Page 240 - MODERN ELECTROCHEMISTRY
P. 240
176 CHAPTER 2
in the gas phase. The interpretation of this is in terms of the structured presence of
water molecules around the ion (low entropy). However, there must be another
component in the events that make up the measured entropy, for the ion breaks the
water structure; i.e., it increases entropy. This is called the “hydrophobic aspect of
solvation.” There is a large literature on this phenomenon and it can be seen by its
effects on several properties of solutions, not only on and but also on the
partial molar volume, specific heat effect, etc.
Among the early discussions of hydrophobic effects were those of Frank et al.
They studied the highly negative entropies of hydration of the rare-gas atoms.
These might have been expected to give much less negative values because of the
absence of tightly ordered hydration shells. To interpret the order indicated by the
highly negative entropies, they suggested that when the normal structure of water was
broken down by the dissolution of the rare-gas atoms, a new type of water structure—
iceberglike groups—was formed. Such groups arise from the breakup of normal water
and thus result from hydrophobicity.
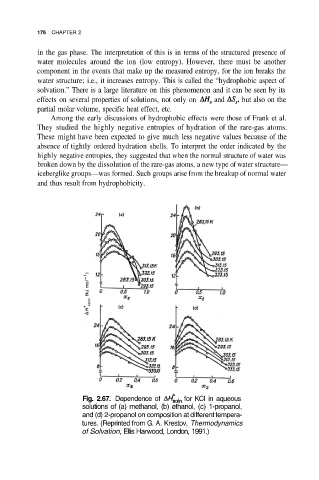
Fig. 2.67. Dependence of for KCI in aqueous
solutions of (a) methanol, (b) ethanol, (c) 1-propanol,
and (d) 2-propanol on composition at different tempera-
tures. (Reprinted from G. A. Krestov, Thermodynamics
of Solvation, Ellis Harwood, London, 1991.)