Page 242 - MODERN ELECTROCHEMISTRY
P. 242
178 CHAPTER 2
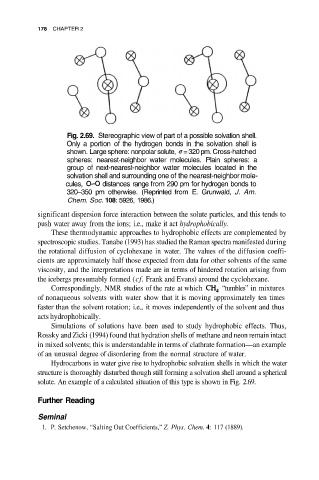
Fig. 2.69. Stereographic view of part of a possible solvation shell.
Only a portion of the hydrogen bonds in the solvation shell is
shown. Large sphere: nonpolar solute, =320pm. Cross-hatched
spheres: nearest-neighbor water molecules. Plain spheres: a
group of next-nearest-neighbor water molecules located in the
solvation shell and surrounding one of the nearest-neighbor mole-
cules, distances range from 290 pm for hydrogen bonds to
320–350 pm otherwise. (Reprinted from E. Grunwald, J. Am.
Chem. Soc. 108: 5926, 1986.)
significant dispersion force interaction between the solute particles, and this tends to
push water away from the ions; i.e., make it act hydrophobically.
These thermodynamic approaches to hydrophobic effects are complemented by
spectroscopic studies. Tanabe (1993) has studied the Raman spectra manifested during
the rotational diffusion of cyclohexane in water. The values of the diffusion coeffi-
cients are approximately half those expected from data for other solvents of the same
viscosity, and the interpretations made are in terms of hindered rotation arising from
the icebergs presumably formed (cf. Frank and Evans) around the cyclohexane.
Correspondingly, NMR studies of the rate at which “tumbles” in mixtures
of nonaqueous solvents with water show that it is moving approximately ten times
faster than the solvent rotation; i.e., it moves independently of the solvent and thus
acts hydrophobically.
Simulations of solutions have been used to study hydrophobic effects. Thus,
Rossky and Zicki (1994) found that hydration shells of methane and neon remain intact
in mixed solvents; this is understandable in terms of clathrate formation—an example
of an unusual degree of disordering from the normal structure of water.
Hydrocarbons in water give rise to hydrophobic solvation shells in which the water
structure is thoroughly disturbed though still forming a solvation shell around a spherical
solute. An example of a calculated situation of this type is shown in Fig. 2.69.
Further Reading
Seminal
1. P. Setchenow, “Salting Out Coefficients,” Z. Phys. Chem. 4: 117 (1889).