Page 247 - MODERN ELECTROCHEMISTRY
P. 247
ION–SOLVENT INTERACTIONS 183
bond and hence destroy its dielectric properties. Thus, is the force on a dipole,
where Xis the electric fieldand is the dipole moment. If for the liquid
is the standard free energy of a dissociation reaction), it should break down.
The results ofcalculations along these lines for water show that more than
would be needed and this kind of electric field strength could only be attained at an
interface. However, even then, breakdown by electrical tearing apart does not merit
too much attention any more because it has been known since the 1950s that fields at
interfaces between electrodes and solutions are on the order of (Chapter
6), yet water there retains its chemical stability.
On the other hand, most chemists and physicists who have discussed this phe-
nomenon in the 1980s and 1990s observed that the streamers come from the electrode
and that light is emitted from the electrode. The interfacial region undoubtedly plays
the determinative role in the dielectric breakdown of liquids.
One view has concentrated upon seeing water and dilute solutions thereof as if
they were intrinsic semiconductors, i.e., semiconductors in which no impurities have
been added to provide foreign atoms that could ionize and provide electrons to increase
conductance. Such bodies are known to have three vital regions. In one, the electron
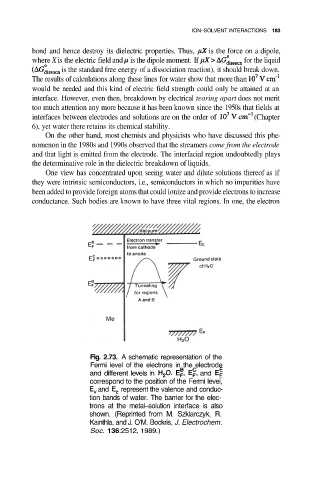
Fig. 2.73. A schematic representation of the
Fermi level of the electrons in the electrode
and different levels in and
correspond to the position of the Fermi level,
and represent the valence and conduc-
tion bands of water. The barrier for the elec-
trons at the metal–solution interface is also
shown. (Reprinted from M. Szklarczyk, R.
Kainthla, and J. O’M. Bockris, J. Electrochem.
Soc. 136:2512, 1989.)