Page 241 - MODERN ELECTROCHEMISTRY
P. 241
ION–SOLVENT INTERACTIONS 177
Frank and Evans, in studying the numbers for the hydrational entropies of ordinary
monatomic ions, found them insufficiently negative, indicating that, due to structure
breaking, the entropy of the ion itself should be larger than that expected if only the
ordering effect of the ion is considered.
An interesting variation of the heat of solution (e.g., for KC1) can be observed in
water–alcohol mixtures. The position of the maxima of the curves shifts with increas-
ing temperature along the ordinate in conjunction with the decrease of the endother-
micity of the KC1 dissolution. This is related to an increasing disruption of the water
structure—a hydrophobic effect (Fig. 2.67).
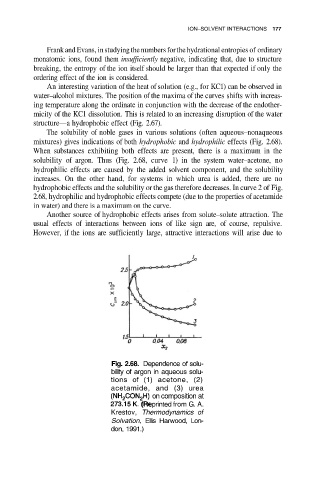
The solubility of noble gases in various solutions (often aqueous–nonaqueous
mixtures) gives indications of both hydrophobic and hydrophilic effects (Fig. 2.68).
When substances exhibiting both effects are present, there is a maximum in the
solubility of argon. Thus (Fig. 2.68, curve 1) in the system water–acetone, no
hydrophilic effects are caused by the added solvent component, and the solubility
increases. On the other hand, for systems in which urea is added, there are no
hydrophobic effects and the solubility or the gas therefore decreases. In curve 2 of Fig.
2.68, hydrophilic and hydrophobic effects compete (due to the properties of acetamide
in water) and there is a maximum on the curve.
Another source of hydrophobic effects arises from solute–solute attraction. The
usual effects of interactions between ions of like sign are, of course, repulsive.
However, if the ions are sufficiently large, attractive interactions will arise due to
Fig. 2.68. Dependence of solu-
bility of argon in aqueous solu-
tions of (1) acetone, (2)
acetamide, and (3) urea
on composition at
(Reprinted from G. A.
Krestov, Thermodynamics of
Solvation, Ellis Harwood, Lon-
don, 1991.)