Page 271 - MODERN ELECTROCHEMISTRY
P. 271
ION–SOLVENT INTERACTIONS 207
and subatomic charged bodies. However, there is a greater doubt. What is required in
calculations of solvation energy is the ion–solvent interaction energy. Does the Born
equation measure the difference of two self-energies, which is not a quantity to be used
in solvation calculations at all?
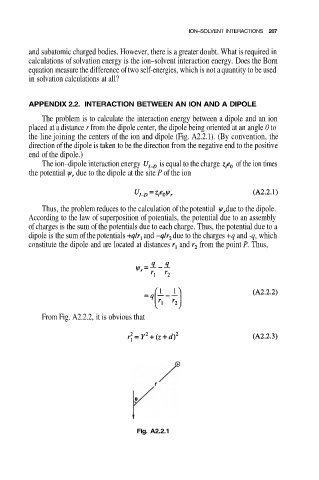
APPENDIX 2.2. INTERACTION BETWEEN AN ION AND A DIPOLE
The problem is to calculate the interaction energy between a dipole and an ion
placed at a distance r from the dipole center, the dipole being oriented at an angle 0 to
the line joining the centers of the ion and dipole (Fig. A2.2.1). (By convention, the
direction of the dipole is taken to be the direction from the negative end to the positive
end of the dipole.)
The ion–dipole interaction energy is equal to the charge of the ion times
the potential due to the dipole at the site P of the ion
Thus, the problem reduces to the calculation of the potential due to the dipole.
According to the law of superposition of potentials, the potential due to an assembly
of charges is the sum of the potentials due to each charge. Thus, the potential due to a
dipole is the sum of the potentials and due to the charges +q and -q, which
constitute the dipole and are located at distances and from the point P. Thus,
From Fig. A2.2.2, it is obvious that
Fig. A2.2.1