Page 298 - MODERN ELECTROCHEMISTRY
P. 298
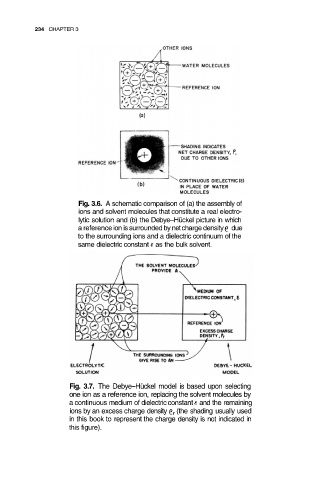
234 CHAPTER 3
Fig. 3.6. A schematic comparison of (a) the assembly of
ions and solvent molecules that constitute a real electro-
lytic solution and (b) the Debye–Hückel picture in which
a reference ion is surrounded by net charge density due
to the surrounding ions and a dielectric continuum of the
same dielectric constant as the bulk solvent.
Fig. 3.7. The Debye–Hückel model is based upon selecting
one ion as a reference ion, replacing the solvent molecules by
a continuous medium of dielectric constant and the remaining
ions by an excess charge density (the shading usually used
in this book to represent the charge density is not indicated in
this figure).