Page 295 - MODERN ELECTROCHEMISTRY
P. 295
ION–ION INTERACTIONS 231
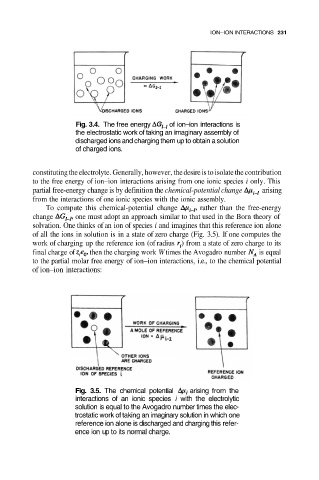
Fig. 3.4. The free energy of ion–ion interactions is
the electrostatic work of taking an imaginary assembly of
discharged ions and charging them up to obtain a solution
of charged ions.
constituting the electrolyte. Generally, however, the desire is to isolate the contribution
to the free energy of ion–ion interactions arising from one ionic species i only. This
partial free-energy change is by definition the chemical-potential change arising
from the interactions of one ionic species with the ionic assembly.
To compute this chemical-potential change rather than the free-energy
change one must adopt an approach similar to that used in the Born theory of
solvation. One thinks of an ion of species i and imagines that this reference ion alone
of all the ions in solution is in a state of zero charge (Fig. 3.5). If one computes the
work of charging up the reference ion (of radius ) from a state of zero charge to its
final charge of then the charging work W times the Avogadro number is equal
to the partial molar free energy of ion–ion interactions, i.e., to the chemical potential
of ion–ion interactions:
Fig. 3.5. The chemical potential arising from the
interactions of an ionic species i with the electrolytic
solution is equal to the Avogadro number times the elec-
trostatic work of taking an imaginary solution in which one
reference ion alone is discharged and charging this refer-
ence ion up to its normal charge.