Page 292 - MODERN ELECTROCHEMISTRY
P. 292
228 CHAPTER 3
The result of the proton transfer is that two ions have been produced: (1) an acetate
ion and (2) a hydrated proton. Thus, potential electrolytes (organic acids and most
bases) dissociate into ions by ionogenic, or ion-forming, chemical reactions with
solvent molecules, in contrast to true electrolytes, which often give rise to ionic
solutions by physical interactions between ions present in the ionic crystal and solvent
molecules (Fig. 3.1).
3.2.3. An Obsolete Classification: Strong and Weak Electrolytes
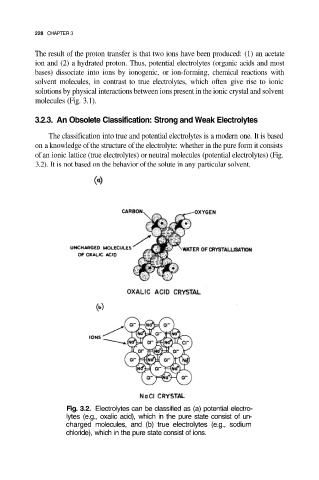
The classification into true and potential electrolytes is a modern one. It is based
on a knowledge of the structure of the electrolyte: whether in the pure form it consists
of an ionic lattice (true electrolytes) or neutral molecules (potential electrolytes) (Fig.
3.2). It is not based on the behavior of the solute in any particular solvent.
Fig. 3.2. Electrolytes can be classified as (a) potential electro-
lytes (e.g., oxalic acid), which in the pure state consist of un-
charged molecules, and (b) true electrolytes (e.g., sodium
chloride), which in the pure state consist of ions.