Page 287 - MODERN ELECTROCHEMISTRY
P. 287
ION–SOLVENT INTERACTIONS 223
in which the thermal energy, RT, is competitive with the electric interaction.
(Mussini)
26. The heat of hydration of an electrolyte is obtained by measuring the heat of
dissolution at various concentrations and extrapolating its values to
0. This value is then used in conjunction with the lattice energy to yield the
desired heat. Explain, then, how the free energy (hence the entropy) of the
hydration of an electrolyte is obtained. Apart from the heat of solution, what
other essential measurements would be necessary?
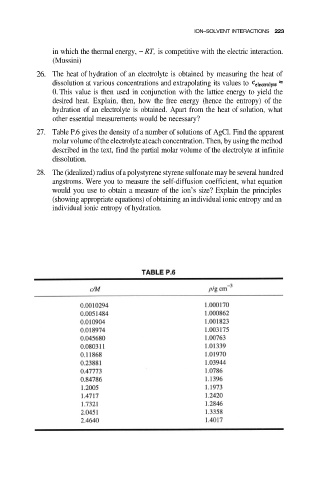
27. Table P.6 gives the density of a number of solutions of AgCl. Find the apparent
molar volume of the electrolyte at each concentration. Then, by using the method
described in the text, find the partial molar volume of the electrolyte at infinite
dissolution.
28. The (idealized) radius of a polystyrene styrene sulfonate may be several hundred
angstroms. Were you to measure the self-diffusion coefficient, what equation
would you use to obtain a measure of the ion’s size? Explain the principles
(showing appropriate equations) of obtaining an individual ionic entropy and an
individual ionic entropy of hydration.