Page 285 - MODERN ELECTROCHEMISTRY
P. 285
ION–SOLVENT INTERACTIONS 221
21. (a) Justify that in a dilute solution solvation number
electrolytes. (Xu)
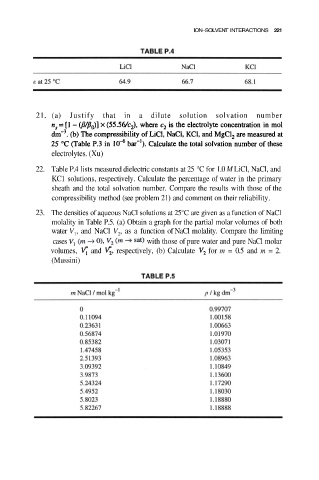
22. Table P.4 lists measured dielectric constants at 25 °C for 1.0 M LiCl, NaCl, and
KC1 solutions, respectively. Calculate the percentage of water in the primary
sheath and the total solvation number. Compare the results with those of the
compressibility method (see problem 21) and comment on their reliability.
23. The densities of aqueous NaCl solutions at 25°C are given as a function of NaCl
molality in Table P.5. (a) Obtain a graph for the partial molar volumes of both
water V , and NaCl V , as a function of NaCl molality. Compare the limiting
1 2
cases with those of pure water and pure NaCl molar
volumes, and respectively, (b) Calculate for m = 0.5 and m = 2.
(Mussini)