Page 284 - MODERN ELECTROCHEMISTRY
P. 284
220 CHAPTER 2
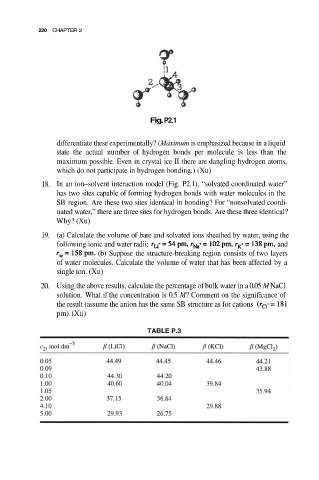
Fig. P2.1
differentiate these experimentally? (Maximum is emphasized because in a liquid
state the actual number of hydrogen bonds per molecule is less than the
maximum possible. Even in crystal ice II there are dangling hydrogen atoms,
which do not participate in hydrogen bonding.) (Xu)
18. In an ion–solvent interaction model (Fig. P2.1), “solvated coordinated water”
has two sites capable of forming hydrogen bonds with water molecules in the
SB region. Are these two sites identical in bonding? For “nonsolvated coordi-
nated water,” there are three sites for hydrogen bonds. Are these three identical?
Why? (Xu)
19. (a) Calculate the volume of bare and solvated ions sheathed by water, using the
following ionic and water radii: and
(b) Suppose the structure-breaking region consists of two layers
of water molecules. Calculate the volume of water that has been affected by a
single ion. (Xu)
20. Using the above results, calculate the percentage of bulk water in a 0.05 M NaCl
solution. What if the concentration is 0.5 M? Comment on the significance of
the result (assume the anion has the same SB structure as for cations
pm). (Xu)