Page 101 - Modern physical chemistry
P. 101
5.6 Entropy of Mixing 91
Since the surrounding material behaves reversibly, for it we have
[5.31 ]
Adding (5.30) and (5.31) gives us
dS ~ (~ -~) dql = T2 -11 dql ~ 0 [5.32]
11 T2 11T2
for the total entropy change. The last inequality follows from the zeroth law ..
The results we have obtained are embodied in the second law of thermodynamics:
For a uniform system in contact with a reversibly acting surroundings, there is a func-
tion of state, entropy S, whose changes are governed by
dS~ dq. [5.33]
T
The total entropy change of an isolated system is positive for a spontaneous change.
5.6 Entropy of Mixing
The essential features of a spontaneous process are exhibited in the mixing of two
ideal gases to form an ideal solution.
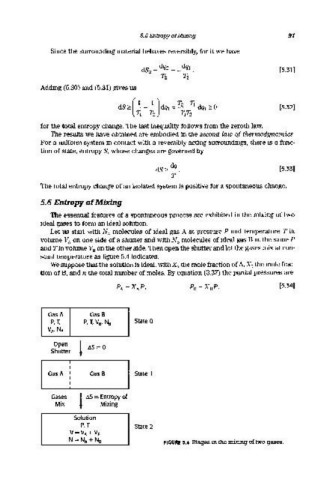
Let us start with NA molecules of ideal gas A at pressure P and temperature T in
volume VA on one side of a shutter and with NB molecules of ideal gas B at the same P
and Tin volume VB on the other side. Then open the shutter and let the gases mix at con-
stant temperature as figure 5.4 indicates.
We suppose that the solution is ideal, with X A the mole fraction of A, X B the mole frac-
tion of B, and n the total number of moles. By equation (3.37) the partial pressures are
[5.34]
Gas A Gas B
P, T, P, T, VB< NB State 0
VAt NA
Open ! ~S=O
Shutter
Gas A Gas B State 1
i
Gases ! ~S = E~t~opy of
Mix MIXing
Solution
P, T State 2
V=VA + VB
N = NA + NB FIGURE 5.4 Stages in the mixing of two gases.