Page 15 - Modern physical chemistry
P. 15
4 Structure in Solids
----.. --
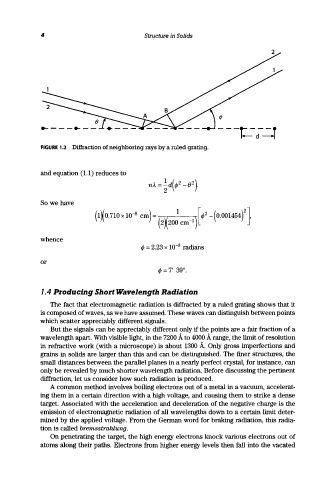
FIGURE 1.2 Diffraction of neighboring rays by a ruled grating.
and equation (1.1) reduces to
So we have
whence
4> = 2.23 x 10- 3 radians
or
4> = 7' 39".
1.4 Producing Short Wavelength Radiation
The fact that electromagnetic radiation is diffracted by a ruled grating shows that it
is composed of waves, as we have assumed. These waves can distinguish between points
which scatter appreciably different signals.
But the signals can be appreciably different only if the points are a fair fraction of a
wavelength apart. With visible light, in the 7200 A to 4000 A range, the limit of resolution
in refractive work (with a microscope) is about 1300 A. Only gross imperfections and
grains in solids are larger than this and can be distinguished. The finer structures, the
small distances between the parallel planes in a nearly perfect crystal, for instance, can
only be revealed by much shorter wavelength radiation. Before discussing the pertinent
diffraction, let us consider how such radiation is produced.
A cornmon method involves boiling electrons out of a metal in a vacuum, accelerat-
ing them in a certain direction with a high voltage, and causing them to strike a dense
target. Associated with the acceleration and deceleration of the negative charge is the
emission of electromagnetic radiation of all wavelengths down to a certain limit deter-
mined by the applied voltage. From the German word for braking radiation, this radia-
tion is called bremsstrahlung.
On penetrating the target, the high energy electrons knock various electrons out of
atoms along their paths. Electrons from higher energy levels then fall into the vacated