Page 378 - Multidimensional Chromatography
P. 378
Multidimensional Chromatography in Environmental Analysis 369
This is a highly efficient barrier against evaporative losses of volatile compounds,
which also improves the peak width of the early eluting compounds. This system has
been successfully applied to a group of pesticides, using n-decane as the co-solvent
and has enabled a group of volatile phosphorus pesticides to be determined (95). The
experimental conditions used in this work are shown in Table 13.2.
Another way of introducing water directly into a gas chromatography system, and
avoiding the need for a retention gap, is to use thermal desorption instead of solvent
desorption, namely on-line solid-phase extraction–thermal desorption (SPETD)
(104). This system has been used in environmental analysis with good results
(105,106). A PTV injector is filled with an SPE sorbent in which the analytes in
water are retained. The choice of sorbent is very important and several different ones
have been tested (107). For instance, when injecting 500 l of water sample into a
PTV liner filled with Tenax TA, the detection limits are between 0.01 g l 1 (for
dieldrin, using an ECD) and 0.5 g l 1 (for aldimorph, using an NPD) (106). As no
sample preparation steps are required with this system, the chances of contamination
are reduced and substances with a high water solubility can be enriched because
such enrichment takes place out of the gas phase. The major drawback with this sys-
tem is the long injection time because the injection rate must be below the evapora-
tion rate. An improved set-up has been used to analyse surface water and tap water
samples (108). The void volume of the injector was reduced and a make-up gas was
added in the backflush mode in order to prevent water from reaching the analytical
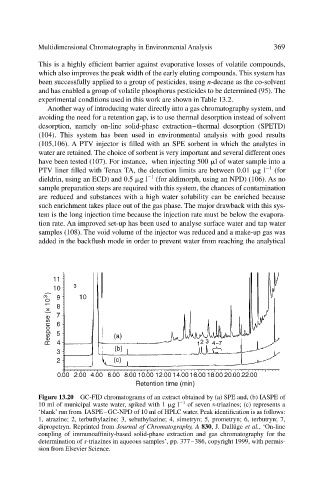
Figure 13.20 GC-FID chromatograms of an extract obtained by (a) SPE and, (b) IASPE of
10 ml of municipal waste water, spiked with 1 g l 1 of seven s-triazines; (c) represents a
‘blank’run from IASPE–GC-NPD of 10 ml of HPLC water. Peak identification is as follows:
1, atrazine; 2, terbuthylazine; 3, sebuthylazine; 4, simetryn; 5, prometryn; 6, terbutryn; 7,
dipropetryn. Reprinted from Journal of Chromatography, A 830, J. Dallüge et al., ‘On-line
coupling of immunoaffinity-based solid-phase extraction and gas chromatography for the
determination of s-triazines in aqueous samples’, pp. 377–386, copyright 1999, with permis-
sion from Elsevier Science.