Page 256 - Multifunctional Photocatalytic Materials for Energy
P. 256
Multidimensional TiO 2 nanostructured catalysts for sustainable H 2 generation 239
a mixture (100 mL) of 10 mL titanium ammonium lactate dihydroxide aqueous solu-
tion (50%), 0.1 M urea, and additional distilled water were prepared and transferred to
a Teflon-lined autoclave and kept at 160°C for 24 h. After that, the mixture was cooled
in air, followed by centrifugation to clear the precipitates and reach a pH value of 7.
For rutile TiO 2 , titanium tetrachloride was added to ethanol dropwise with continuous
stirring. When a transparent yellowish sol formed, the resulting solution was slowly
added to distilled water and stirred for another 30 min, and then the resultants were
maintained at 50°C in an oven for 24 h. After centrifugation, the samples were washed
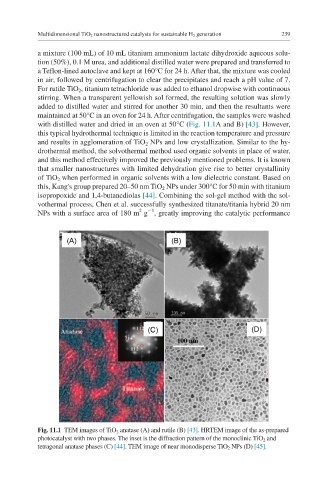
with distilled water and dried in an oven at 50°C (Fig. 11.1A and B) [43]. However,
this typical hydrothermal technique is limited in the reaction temperature and pressure
and results in agglomeration of TiO 2 NPs and low crystallization. Similar to the hy-
drothermal method, the solvothermal method used organic solvents in place of water,
and this method effectively improved the previously mentioned problems. It is known
that smaller nanostructures with limited dehydration give rise to better crystallinity
of TiO 2 when performed in organic solvents with a low dielectric constant. Based on
this, Kang's group prepared 20–50 nm TiO 2 NPs under 300°C for 50 min with titanium
isopropoxide and 1,4-butanediolas [44]. Combining the sol-gel method with the sol-
vothermal process, Chen et al. successfully synthesized titanate/titania hybrid 20 nm
− 1
2
NPs with a surface area of 180 m g , greatly improving the catalytic performance
Fig. 11.1 TEM images of TiO 2 anatase (A) and rutile (B) [43]. HRTEM image of the as-prepared
photocatalyst with two phases. The inset is the diffraction pattern of the monoclinic TiO 2 and
tetragonal anatase phases (C) [44]. TEM image of near monodisperse TiO 2 NPs (D) [45].