Page 278 - Multifunctional Photocatalytic Materials for Energy
P. 278
260
8
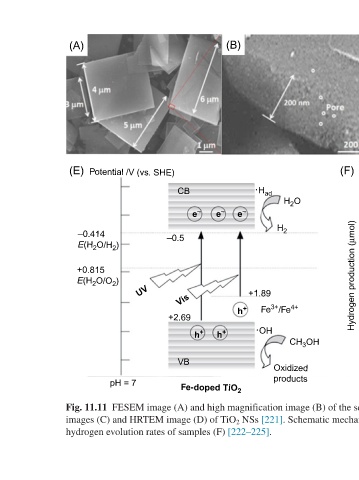
FESEM image (A) and high magnification image (B) of the selected red marked part of (A), STEM-EDS elemental mapping
7
6
5
(D)
Time (h)
4
3
10.95
0.83
0.97
2
S 5
S 4
S 3
1
(C)
0
20
10
0
40
50
30
(F) 80 rate samples (µmol h –1 ) 70 S 0 1.40 1.68 S 1 3.47 S 2 60 Multifunctional Photocatalytic Materials for Energy images (C) and HRTEM image (D) of TiO 2 NSs [221]. Schematic mechanism for the hydrogen production of Fe-doped TiO 2 (E) and the
Hydrogen production (µmol)
CH 3 OH
H 2 O Oxidized products
H 2 Fe 3+ /Fe 4+
⋅H ad +1.89 ⋅OH
e – h +
(B)
e – h + Fe-doped TiO 2
e – h +
CB –0.5 Vis +2.69 VB hydrogen evolution rates of samples (F) [222–225].
Potential /V (vs. SHE) UV pH = 7
(A) (E) –0.414 E(H 2 O/H 2 ) +0.815 E(H 2 O/O 2 ) Fig. 11.11