Page 273 - Multifunctional Photocatalytic Materials for Energy
P. 273
Multidimensional TiO 2 nanostructured catalysts for sustainable H 2 generation 255
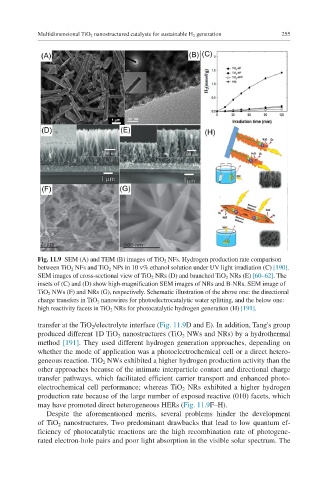
Fig. 11.9 SEM (A) and TEM (B) images of TiO 2 NFs. Hydrogen production rate comparison
between TiO 2 NFs and TiO 2 NPs in 10 v% ethanol solution under UV light irradiation (C) [190].
SEM images of cross-sectional view of TiO 2 NRs (D) and branched TiO 2 NRs (E) [60–62]. The
insets of (C) and (D) show high-magnification SEM images of NRs and B-NRs. SEM image of
TiO 2 NWs (F) and NRs (G), respectively. Schematic illustration of the above one: the directional
charge transfers in TiO 2 nanowires for photoelectrocatalytic water splitting, and the below one:
high reactivity facets in TiO 2 NRs for photocatalytic hydrogen generation (H) [191].
transfer at the TiO 2 /electrolyte interface (Fig. 11.9D and E). In addition, Tang's group
produced different 1D TiO 2 nanostructures (TiO 2 NWs and NRs) by a hydrothermal
method [191]. They used different hydrogen generation approaches, depending on
whether the mode of application was a photoelectrochemical cell or a direct hetero-
geneous reaction. TiO 2 NWs exhibited a higher hydrogen production activity than the
other approaches because of the intimate interparticle contact and directional charge
transfer pathways, which facilitated efficient carrier transport and enhanced photo-
electrochemical cell performance; whereas TiO 2 NRs exhibited a higher hydrogen
production rate because of the large number of exposed reactive (010) facets, which
may have promoted direct heterogeneous HERs (Fig. 11.9F–H).
Despite the aforementioned merits, several problems hinder the development
of TiO 2 nanostructures. Two predominant drawbacks that lead to low quantum ef-
ficiency of photocatalytic reactions are the high recombination rate of photogene-
rated electron- hole pairs and poor light absorption in the visible solar spectrum. The