Page 46 - Multifunctional Photocatalytic Materials for Energy
P. 46
Metal oxide electrodes for photo-activated water splitting 35
(B) 0.5
50th cycle
(A) 0.4
1st cycle
J (mA × cm –2 ) 0.3 GQDs@ZnO NWs_light
0.2
0.1 ZnO NWs_light
ZnO NWs_dark
GQDs@ZnO NWs_dark
0.0
–0.2 0.0 0.2 0.4 0.6 0.8 1.0
Voltage (V) vs. RHE
(C)
H 2
Sunlight
3.5 eV Platinum
H 2 O
FTO 4.2 eV
4.7 eV H 2 O
GQD
ZnO
5.4 eV
O 2
7.5 eV
Aqueouselectrolyte
GDQ@ZnONW
Semiconductor photoanode
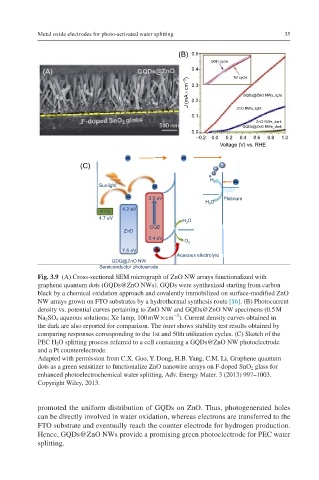
Fig. 3.9 (A) Cross-sectional SEM micrograph of ZnO NW arrays functionalized with
graphene quantum dots (GQDs@ZnO NWs). GQDs were synthesized starting from carbon
black by a chemical oxidation approach and covalently immobilized on surface-modified ZnO
NW arrays grown on FTO substrates by a hydrothermal synthesis route [16]. (B) Photocurrent
density vs. potential curves pertaining to ZnO NW and GQDs@ZnO NW specimens (0.5 M
−2
Na 2 SO 4 aqueous solutions; Xe lamp, 100 mW × cm ). Current density curves obtained in
the dark are also reported for comparison. The inset shows stability test results obtained by
comparing responses corresponding to the 1st and 50th utilization cycles. (C) Sketch of the
PEC H 2 O splitting process referred to a cell containing a GQDs@ZnO NW photoelectrode
and a Pt counterelectrode.
Adapted with permission from C.X. Guo, Y. Dong, H.B. Yang, C.M. Li, Graphene quantum
dots as a green sensitizer to functionalize ZnO nanowire arrays on F-doped SnO 2 glass for
enhanced photoelectrochemical water splitting, Adv. Energy Mater. 3 (2013) 997–1003.
Copyright Wiley, 2013.
promoted the uniform distribution of GQDs on ZnO. Thus, photogenerated holes
can be directly involved in water oxidation, whereas electrons are transferred to the
FTO substrate and eventually reach the counter electrode for hydrogen production.
Hence, GQDs@ZnO NWs provide a promising green photoelectrode for PEC water
splitting.