Page 30 - Book Hosokawa Nanoparticle Technology Handbook
P. 30
1.1 SIZE EFFECT AND PROPERTIES OF NANOPARTICLES FUNDAMENTALS
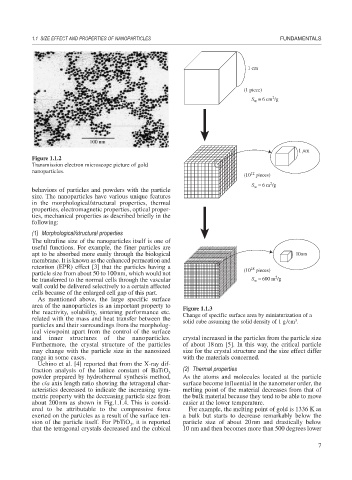
1 cm
(1 piece)
2
= 6 cm /g
S w
1 m
Figure 1.1.2
Transmission electron microscope picture of gold
nanoparticles.
12
(10 pieces)
2
S = 6 m /g
w
behaviors of particles and powders with the particle
size. The nanoparticles have various unique features
in the morphological/structural properties, thermal
properties, electromagnetic properties, optical proper-
ties, mechanical properties as described briefly in the
following:
(1) Morphological/structural properties
The ultrafine size of the nanoparticles itself is one of
useful functions. For example, the finer particles are
apt to be absorbed more easily through the biological 10nm
membrane. It is known as the enhanced permeation and
retention (EPR) effect [3] that the particles having a (10 pieces)
18
particle size from about 50 to 100nm, which would not
2
be transferred to the normal cells through the vascular S = 600 m /g
w
wall could be delivered selectively to a certain affected
cells because of the enlarged cell gap of this part.
As mentioned above, the large specific surface
area of the nanoparticles is an important property to Figure 1.1.3
the reactivity, solubility, sintering performance etc. Change of specific surface area by miniaturization of a
related with the mass and heat transfer between the solid cube assuming the solid density of 1 g/cm .
3
particles and their surroundings from the morpholog-
ical viewpoint apart from the control of the surface
and inner structures of the nanoparticles. crystal increased in the particles from the particle size
Furthermore, the crystal structure of the particles of about 18nm [5]. In this way, the critical particle
may change with the particle size in the nanosized size for the crystal structure and the size effect differ
range in some cases. with the materials concerned.
Uchino et al. [4] reported that from the X-ray dif-
fraction analysis of the lattice constant of BaTiO 3 (2) Thermal properties
powder prepared by hydrothermal synthesis method, As the atoms and molecules located at the particle
the c/a axis length ratio showing the tetragonal char- surface become influential in the nanometer order, the
acteristics decreased to indicate the increasing sym- melting point of the material decreases from that of
metric property with the decreasing particle size from the bulk material because they tend to be able to move
about 200nm as shown in Fig.1.1.4. This is consid- easier at the lower temperature.
ered to be attributable to the compressive force For example, the melting point of gold is 1336 K as
exerted on the particles as a result of the surface ten- a bulk but starts to decrease remarkably below the
sion of the particle itself. For PbTiO , it is reported particle size of about 20nm and drastically below
3
that the tetragonal crystals decreased and the cubical 10 nm and then becomes more than 500 degrees lower
7