Page 31 - Book Hosokawa Nanoparticle Technology Handbook
P. 31
FUNDAMENTALS CH. 1 BASIC PROPERTIES AND MEASURING METHODS OF NANOPARTICLES
Table 1.1.1
Solid particle size and the fraction of atoms located at the particle surface.
Number of Number of Total Number ratio of Examples of
atoms atoms number surface atoms particle size
in a side at the surface of atoms to the total (%) and powder
2 8 8 100
3 26 27 97
4 56 64 87.5
5 98 125 78.5
10 488 1,000 48.8 2nm
100 58,800 1 10 6 5.9 20nm (colloidal silica)
1,000 6 10 6 1 10 9 0.6 200nm (titanium dioxide)
10,000 6 10 8 1 10 12 0.06 2 m (light calcium carbonate)
100,000 6 10 10 1 10 15 0.006 20 m (green tea powder, chalk)
1 m 1 10 m =1 10 nm that when the dielectric constant is measured with a
9
6
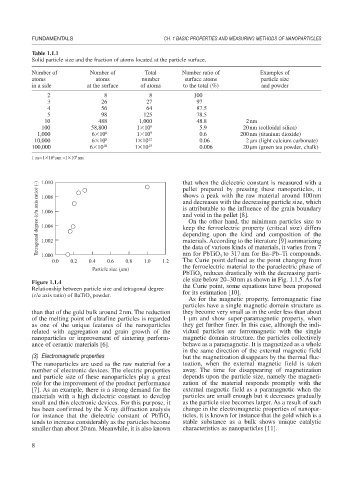
1.010
Tetragonal degree (c/a axis ratio) (-) 1.008 pellet prepared by pressing these nanoparticles, it
shows a peak with the raw material around 100nm
and decreases with the decreasing particle size, which
is attributable to the influence of the grain boundary
1.006
and void in the pellet [8].
On the other hand, the minimum particles size to
1.004
keep the ferroelectric property (critical size) differs
depending upon the kind and composition of the
1.002
the data of various kinds of materials, it varies from 7
nm for PbTiO to 317nm for Ba–Pb–Ti compounds.
1.000
3
The Curie point defined as the point changing from
0.0 0.2 0.4 0.6 0.8 1.0 1.2 materials. According to the literature [9] summarizing
the ferroelectric material to the paraelectric phase of
Particle size ( m)
PbTiO reduces drastically with the decreasing parti-
3
cle size below 20–30nm as shown in Fig. 1.1.5. As for
Figure 1.1.4 the Curie point, some equations have been proposed
Relationship between particle size and tetragonal degree for its estimation [10].
(c/a axis ratio) of BaTiO powder. As for the magnetic property, ferromagnetic fine
3
particles have a single magnetic domain structure as
than that of the gold bulk around 2nm. The reduction they become very small as in the order less than about
of the melting point of ultrafine particles is regarded 1 m and show super-paramagnetic property, when
as one of the unique features of the nanoparticles they get further finer. In this case, although the indi-
related with aggregation and grain growth of the vidual particles are ferromagnetic with the single
nanoparticles or improvement of sintering perform- magnetic domain structure, the particles collectively
ance of ceramic materials [6]. behave as a paramagnetic. It is magnetized as a whole
in the same direction of the external magnetic field
(3) Electromagnetic properties but the magnetization disappears by the thermal fluc-
The nanoparticles are used as the raw material for a tuation, when the external magnetic field is taken
number of electronic devices. The electric properties away. The time for disappearing of magnetization
and particle size of these nanoparticles play a great depends upon the particle size, namely the magneti-
role for the improvement of the product performance zation of the material responds promptly with the
[7]. As an example, there is a strong demand for the external magnetic field as a paramagnetic when the
materials with a high dielectric constant to develop particles are small enough but it decreases gradually
small and thin electronic devices. For this purpose, it as the particle size becomes larger. As a result of such
has been confirmed by the X-ray diffraction analysis change in the electromagnetic properties of nanopar-
for instance that the dielectric constant of PbTiO 3 ticles, it is known for instance that the gold which is a
tends to increase considerably as the particles become stable substance as a bulk shows unique catalytic
smaller than about 20nm. Meanwhile, it is also known characteristics as nanoparticles [11].
8