Page 65 - Nanotechnology an introduction
P. 65
Figure 6.4 Transmission electron micrograph of cadmium sulfide nanoparticles prepared in the author's laboratory by nucleation and growth in aqueous solution [135]. The apparent agglomeration is an artifact of the sample preparation, in
which a drop of the particle suspension was placed on a carbon-coated copper grid and the water allowed to evaporate. This final stage of the evaporation process can be observed in situ in the electron ultramicroscope: the water forms a
receding thin film and capillary forces (Section 3.3) at its edge drag the particles together.
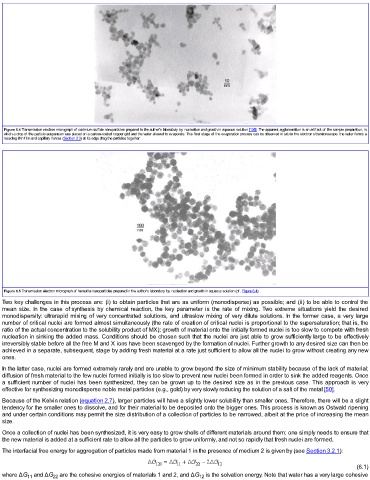
Figure 6.5 Transmission electron micrograph of hematite nanoparticles prepared in the author's laboratory by nucleation and growth in aqueous solution (cf. Figure 6.4).
Two key challenges in this process are: (i) to obtain particles that are as uniform (monodisperse) as possible; and (ii) to be able to control the
mean size. In the case of synthesis by chemical reaction, the key parameter is the rate of mixing. Two extreme situations yield the desired
monodispersity: ultrarapid mixing of very concentrated solutions, and ultraslow mixing of very dilute solutions. In the former case, a very large
number of critical nuclei are formed almost simultaneously (the rate of creation of critical nuclei is proportional to the supersaturation; that is, the
ratio of the actual concentration to the solubility product of MX); growth of material onto the initially formed nuclei is too slow to compete with fresh
nucleation in sinking the added mass. Conditions should be chosen such that the nuclei are just able to grow sufficiently large to be effectively
irreversibly stable before all the free M and X ions have been scavenged by the formation of nuclei. Further growth to any desired size can then be
achieved in a separate, subsequent, stage by adding fresh material at a rate just sufficient to allow all the nuclei to grow without creating any new
ones.
In the latter case, nuclei are formed extremely rarely and are unable to grow beyond the size of minimum stability because of the lack of material;
diffusion of fresh material to the few nuclei formed initially is too slow to prevent new nuclei been formed in order to sink the added reagents. Once
a sufficient number of nuclei has been synthesized, they can be grown up to the desired size as in the previous case. This approach is very
effective for synthesizing monodisperse noble metal particles (e.g., gold) by very slowly reducing the solution of a salt of the metal [50].
Because of the Kelvin relation (equation 2.7), larger particles will have a slightly lower solubility than smaller ones. Therefore, there will be a slight
tendency for the smaller ones to dissolve, and for their material to be deposited onto the bigger ones. This process is known as Ostwald ripening
and under certain conditions may permit the size distribution of a collection of particles to be narrowed, albeit at the price of increasing the mean
size.
Once a collection of nuclei has been synthesized, it is very easy to grow shells of different materials around them; one simply needs to ensure that
the new material is added at a sufficient rate to allow all the particles to grow uniformly, and not so rapidly that fresh nuclei are formed.
The interfacial free energy for aggregation of particles made from material 1 in the presence of medium 2 is given by (see Section 3.2.1):
(6.1)
where ΔG and ΔG are the cohesive energies of materials 1 and 2, and ΔG is the solvation energy. Note that water has a very large cohesive
12
11
22