Page 116 - New Trends In Coal Conversion
P. 116
Minimization of Hg and trace elements during coal combustion and gasification processes 79
(a) 100%
10%
1%
0%
As Mo Sb Pb Ge W P Ga Sn Zn V Cu Ni Cs Na Be K Rb Cr Ba Al Co Sc Li La Zr Fe Hf Ta Y Cd U Sr Se Mn Mg B Ca F Hg S Cl
(b) 100%
10%
1%
0%
As Mo Sn Ge Pb Sb W Zn P Ga V Cu Cd Ni Co Cs Cr Be Ba Sc Al Li Rb K La Ti Na Zr Hf Fe Y U Sr Ta Mg Mn Se B F Ca Hg S Cl
Fly ash Slag FGD gypsum Filtered water PM OUT- FGD Gas OUT- FGD Slag water
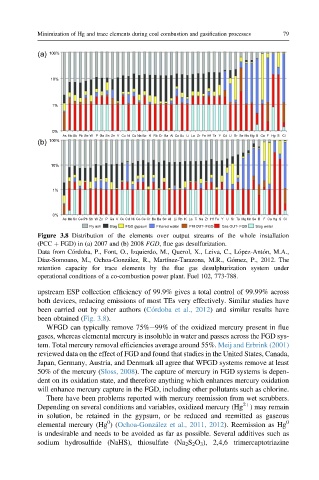
Figure 3.8 Distribution of the elements over output streams of the whole installation
(PCC þ FGD) in (a) 2007 and (b) 2008 FGD, flue gas desulfurization.
Data from C ordoba, P., Font, O., Izquierdo, M., Querol, X., Leiva, C., L opez-Ant on, M.A.,
Díaz-Somoano, M., Ochoa-Gonz alez, R., Martínez-Tarazona, M.R., G omez, P., 2012. The
retention capacity for trace elements by the flue gas desulphurization system under
operational conditions of a co-combustion power plant. Fuel 102, 773-788.
upstream ESP collection efficiency of 99.9% gives a total control of 99.99% across
both devices, reducing emissions of most TEs very effectively. Similar studies have
been carried out by other authors (C ordoba et al., 2012) and similar results have
been obtained (Fig. 3.8).
WFGD can typically remove 75%e99% of the oxidized mercury present in flue
gases, whereas elemental mercury is insoluble in water and passes across the FGD sys-
tem. Total mercury removal efficiencies average around 55%. Meij and Erbrink (2001)
reviewed data on the effect of FGD and found that studies in the United States, Canada,
Japan, Germany, Austria, and Denmark all agree that WFGD systems remove at least
50% of the mercury (Sloss, 2008). The capture of mercury in FGD systems is depen-
dent on its oxidation state, and therefore anything which enhances mercury oxidation
will enhance mercury capture in the FGD, including other pollutants such as chlorine.
There have been problems reported with mercury reemission from wet scrubbers.
2þ
Depending on several conditions and variables, oxidized mercury (Hg ) may remain
in solution, be retained in the gypsum, or be reduced and reemitted as gaseous
0 0
elemental mercury (Hg )(Ochoa-Gonz alez et al., 2011, 2012). Reemission as Hg
is undesirable and needs to be avoided as far as possible. Several additives such as
sodium hydrosulfide (NaHS), thiosulfate (Na 2 S 2 O 3 ), 2,4,6 trimercaptotriazine