Page 427 - Organic Electronics in Sensors and Biotechnology
P. 427
404 Chapter Eleven
1.8
70
1.4 K e – + 60 1.6
Number of transported species (× 10 17 ) 1.0 50 Current (μA) Ratio (a. u.) 1.4
1.2
40
0.8
30
0.6
1.2
20
0.4
0.2
0 10 1.0
0
0.8
0 300 600 900 1,200 1,500 1,800 0 5 10 15 20 25 30 35 40
Time (s) Time (min)
+
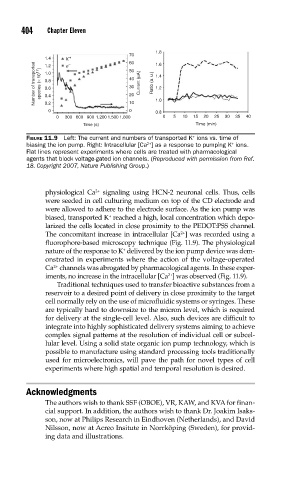
FIGURE 11.9 Left: The current and numbers of transported K ions vs. time of
biasing the ion pump. Right: Intracellular [Ca ] as a response to pumping K ions.
+
2+
Flat lines represent experiments where cells are treated with pharmacological
agents that block voltage-gated ion channels. (Reproduced with permission from Ref.
18. Copyright 2007, Nature Publishing Group.)
2+
physiological Ca signaling using HCN-2 neuronal cells. Thus, cells
were seeded in cell culturing medium on top of the CD electrode and
were allowed to adhere to the electrode surface. As the ion pump was
+
biased, transported K reached a high, local concentration which depo-
larized the cells located in close proximity to the PEDOT:PSS channel.
2+
The concomitant increase in intracellular [Ca ] was recorded using a
fluorophore-based microscopy technique (Fig. 11.9). The physiological
+
nature of the response to K delivered by the ion pump device was dem-
onstrated in experiments where the action of the voltage-operated
2+
Ca channels was abrogated by pharmacological agents. In these exper-
iments, no increase in the intracellular [Ca ] was observed (Fig. 11.9).
2+
Traditional techniques used to transfer bioactive substances from a
reservoir to a desired point of delivery in close proximity to the target
cell normally rely on the use of microfluidic systems or syringes. These
are typically hard to downsize to the micron level, which is required
for delivery at the single-cell level. Also, such devices are difficult to
integrate into highly sophisticated delivery systems aiming to achieve
complex signal patterns at the resolution of individual cell or subcel-
lular level. Using a solid state organic ion pump technology, which is
possible to manufacture using standard processing tools traditionally
used for microelectronics, will pave the path for novel types of cell
experiments where high spatial and temporal resolution is desired.
Acknowledgments
The authors wish to thank SSF (OBOE), VR, KAW, and KVA for finan-
cial support. In addition, the authors wish to thank Dr. Joakim Isaks-
son, now at Philips Research in Eindhoven (Netherlands), and David
Nilsson, now at Acreo Insitute in Norrköping (Sweden), for provid-
ing data and illustrations.