Page 119 - PRINCIPLES OF QUANTUM MECHANICS as Applied to Chemistry and Chemical Physics
P. 119
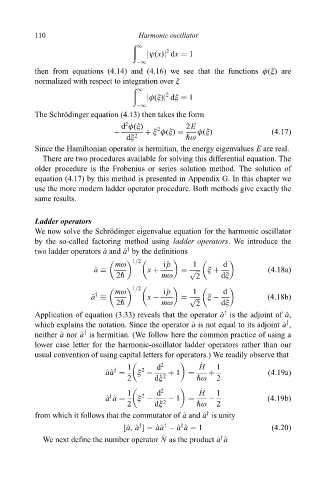
110 Harmonic oscillator
1
2
jø(x)j dx 1
ÿ1
then from equations (4.14) and (4.16) we see that the functions ö(î) are
normalized with respect to integration over î
1
2
jö(î)j dî 1
ÿ1
The Schrodinger equation (4.13) then takes the form
È
2
d ö(î) 2 2E
ÿ î ö(î) ö(î) (4:17)
dî 2 "ù
Since the Hamiltonian operator is hermitian, the energy eigenvalues E are real.
There are two procedures available for solving this differential equation. The
older procedure is the Frobenius or series solution method. The solution of
equation (4.17) by this method is presented in Appendix G. In this chapter we
use the more modern ladder operator procedure. Both methods give exactly the
same results.
Ladder operators
È
We now solve the Schrodinger eigenvalue equation for the harmonic oscillator
by the so-called factoring method using ladder operators. We introduce the
two ladder operators ^ a and ^ a by the de®nitions
y
1=2
mù i^ p 1 d
^ a x p î (4:18a)
2" mù 2 dî
1=2
mù i^ p 1 d
y
^ a x ÿ p î ÿ (4:18b)
2" mù 2 dî
Application of equation (3.33) reveals that the operator ^ a is the adjoint of ^ a,
y
which explains the notation. Since the operator ^ a is not equal to its adjoint ^ a ,
y
neither ^ a nor ^ a is hermitian. (We follow here the common practice of using a
y
lower case letter for the harmonic-oscillator ladder operators rather than our
usual convention of using capital letters for operators.) We readily observe that
^
1 d 2 H 1
y 2
^ a^ a î ÿ 1 (4:19a)
2 dî 2 "ù 2
^
1 d 2 H 1
y 2
^ a ^ a î ÿ ÿ 1 ÿ (4:19b)
2 dî 2 "ù 2
y
from which it follows that the commutator of ^ a and ^ a is unity
y
[^ a, ^ a ] ^ a^ a ÿ ^ a ^ a 1 (4:20)
y
y
^
We next de®ne the number operator N as the product ^ a ^ a
y