Page 215 - PRINCIPLES OF QUANTUM MECHANICS as Applied to Chemistry and Chemical Physics
P. 215
206 Spin
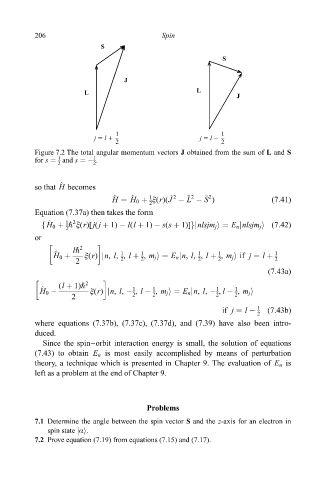
S
S
J
L
L
J
1 1
j 5 l 1 j 5 l 2
2 2
Figure 7.2 The total angular momentum vectors J obtained from the sum of L and S
1
1
for s and s ÿ .
2 2
^
so that H becomes
^
^
^
^
2
^ 2
2
1
H H 0 î(r)(J ÿ L ÿ S ) (7:41)
2
Equation (7.37a) then takes the form
^
1 2
fH 0 " î(r)[j(j 1) ÿ l(l 1) ÿ s(s 1)]gjnlsjm j i E n jnlsjm j i (7:42)
2
or
l" 2
^ 1 1 1 1 1
H 0 î(r) jn, l, , l , m j i E n jn, l, , l , m j i if j l 2
2
2
2
2
2
(7:43a)
(l 1)" 2
^ 1 1 1 1
H 0 ÿ î(r) jn, l, ÿ , l ÿ , m j i E n jn, l, ÿ , l ÿ , m j i
2 2 2 2 2
1
if j l ÿ (7:43b)
2
where equations (7.37b), (7.37c), (7.37d), and (7.39) have also been intro-
duced.
Since the spin±orbit interaction energy is small, the solution of equations
(7.43) to obtain E n is most easily accomplished by means of perturbation
theory, a technique which is presented in Chapter 9. The evaluation of E n is
left as a problem at the end of Chapter 9.
Problems
7.1 Determine the angle between the spin vector S and the z-axis for an electron in
spin state jái.
7.2 Prove equation (7.19) from equations (7.15) and (7.17).