Page 113 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 113
104 ADSORPTION OF VAPORS ON MINERALS AND OTHER SOLIDS
600
Activated carbon-water
Activiated carbon-benzene
Vapor Uptake, Q (mg/g) 400
200
0
0 0.2 0.4 0.6 0.8 1.0
Relative Pressure, P/P°
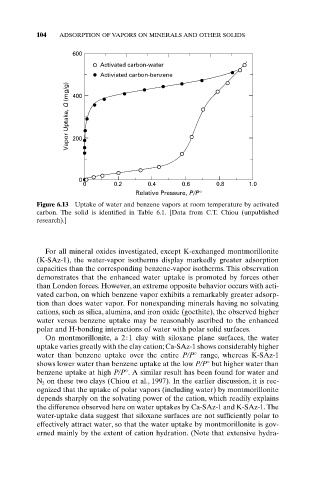
Figure 6.13 Uptake of water and benzene vapors at room temperature by activated
carbon. The solid is identified in Table 6.1. [Data from C.T. Chiou (unpublished
research).]
For all mineral oxides investigated, except K-exchanged montmorillonite
(K-SAz-1), the water-vapor isotherms display markedly greater adsorption
capacities than the corresponding benzene-vapor isotherms. This observation
demonstrates that the enhanced water uptake is promoted by forces other
than London forces. However, an extreme opposite behavior occurs with acti-
vated carbon, on which benzene vapor exhibits a remarkably greater adsorp-
tion than does water vapor. For nonexpanding minerals having no solvating
cations, such as silica, alumina, and iron oxide (goethite), the observed higher
water versus benzene uptake may be reasonably ascribed to the enhanced
polar and H-bonding interactions of water with polar solid surfaces.
On montmorillonite, a 2:1 clay with siloxane plane surfaces, the water
uptake varies greatly with the clay cation; Ca-SAz-1 shows considerably higher
water than benzene uptake over the entire P/P° range, whereas K-SAz-1
shows lower water than benzene uptake at the low P/P° but higher water than
benzene uptake at high P/P°. A similar result has been found for water and
N 2 on these two clays (Chiou et al., 1997). In the earlier discussion, it is rec-
ognized that the uptake of polar vapors (including water) by montmorillonite
depends sharply on the solvating power of the cation, which readily explains
the difference observed here on water uptakes by Ca-SAz-1 and K-SAz-1. The
water-uptake data suggest that siloxane surfaces are not sufficiently polar to
effectively attract water, so that the water uptake by montmorillonite is gov-
erned mainly by the extent of cation hydration. (Note that extensive hydra-