Page 108 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 108
IMPROPER SURFACE-AREA MEASUREMENT 99
soil, which has a ratio of 3.2, the sample is known to contain 1.9% organic
matter and 21% clay of unspecified form; the calculation shows that the effect
of partition into soil organic matter is less significant than the effect of cation
solvation (Chiou et al., 1993).
On the premise that nonpolar organic vapors partition far less efficiently
than polar vapors into soil organic matter, shown in Chapter 7, the analysis
above would suggest that use of the isotherms of nonpolar organic vapors
along with the BET model should give a reasonable estimate of the surface
areas of minerals and low-organic-content soils. This is because these vapors
will not engage in cation solvation and they partition (dissolve) poorly into
relatively polar soil organic matter (Chiou et al., 1993). This point is well man-
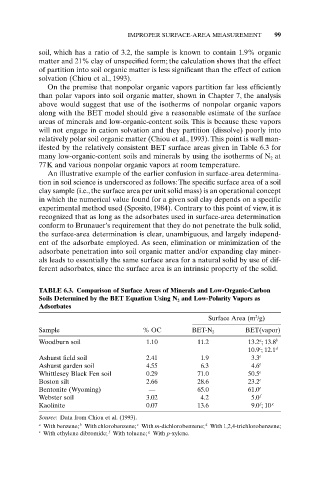
ifested by the relatively consistent BET surface areas given in Table 6.3 for
many low-organic-content soils and minerals by using the isotherms of N 2 at
77K and various nonpolar organic vapors at room temperature.
An illustrative example of the earlier confusion in surface-area determina-
tion in soil science is underscored as follows:The specific surface area of a soil
clay sample (i.e., the surface area per unit solid mass) is an operational concept
in which the numerical value found for a given soil clay depends on a specific
experimental method used (Sposito, 1984). Contrary to this point of view, it is
recognized that as long as the adsorbates used in surface-area determination
conform to Brunauer’s requirement that they do not penetrate the bulk solid,
the surface-area determination is clear, unambiguous, and largely independ-
ent of the adsorbate employed. As seen, elimination or minimization of the
adsorbate penetration into soil organic matter and/or expanding clay miner-
als leads to essentially the same surface area for a natural solid by use of dif-
ferent adsorbates, since the surface area is an intrinsic property of the solid.
TABLE 6.3. Comparison of Surface Areas of Minerals and Low-Organic-Carbon
Soils Determined by the BET Equation Using N 2 and Low-Polarity Vapors as
Adsorbates
2
Surface Area (m /g)
Sample % OC BET-N 2 BET(vapor)
a
Woodburn soil 1.10 11.2 13.2 ; 13.8 b
c
10.9 ; 12.1 d
Ashurst field soil 2.41 1.9 3.3 e
Ashurst garden soil 4.55 6.3 4.6 e
Whittlesey Black Fen soil 0.29 71.0 50.5 e
Boston silt 2.66 28.6 23.2 e
Bentonite (Wyoming) — 65.0 61.0 e
Webster soil 3.02 4.2 5.0 f
f
Kaolinite 0.07 13.6 9.0 ;10 g
Source: Data from Chiou et al. (1993).
b
a With benzene; With chlorobenzene; With m-dichlorobenzene; With 1,2,4-trichlorobenzene;
d
c
e With ethylene dibromide; With toluene; g With p-xylene.
f