Page 105 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 105
96 ADSORPTION OF VAPORS ON MINERALS AND OTHER SOLIDS
Woodburn-EGME
60
Woodburn-nitrogen
2
Vapor Uptake, Q (mg/g) 40 (right scale) 1
Peat-EGME
Peat-nitrogen
20
0 0
0 0.2 0.4 0.6 0.8 1.0
Relative Pressure, P/P°
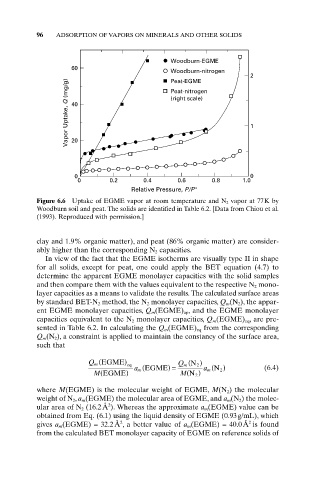
Figure 6.6 Uptake of EGME vapor at room temperature and N 2 vapor at 77K by
Woodburn soil and peat. The solids are identified in Table 6.2. [Data from Chiou et al.
(1993). Reproduced with permission.]
clay and 1.9% organic matter), and peat (86% organic matter) are consider-
ably higher than the corresponding N 2 capacities.
In view of the fact that the EGME isotherms are visually type II in shape
for all solids, except for peat, one could apply the BET equation (4.7) to
determine the apparent EGME monolayer capacities with the solid samples
and then compare them with the values equivalent to the respective N 2 mono-
layer capacities as a means to validate the results. The calculated surface areas
by standard BET-N 2 method, the N 2 monolayer capacities, Q m (N 2 ), the appar-
ent EGME monolayer capacities, Q m (EGME) ap , and the EGME monolayer
capacities equivalent to the N 2 monolayer capacities, Q m (EGME) eq , are pre-
sented in Table 6.2. In calculating the Q m (EGME) eq from the corresponding
Q m (N 2 ), a constraint is applied to maintain the constancy of the surface area,
such that
(
Q m EGME) eq Q m N )
(
2
(
(
a m EGME) = a m N ) (6.4)
2
(
M EGME) M N )
(
2
where M(EGME) is the molecular weight of EGME, M(N 2 ) the molecular
weight of N 2 , a m (EGME) the molecular area of EGME, and a m (N 2 ) the molec-
2
ular area of N 2 (16.2Å ). Whereas the approximate a m (EGME) value can be
obtained from Eq. (6.1) using the liquid density of EGME (0.93g/mL), which
2
2
gives a m (EGME) = 32.2Å , a better value of a m (EGME) = 40.0Å is found
from the calculated BET monolayer capacity of EGME on reference solids of