Page 33 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 33
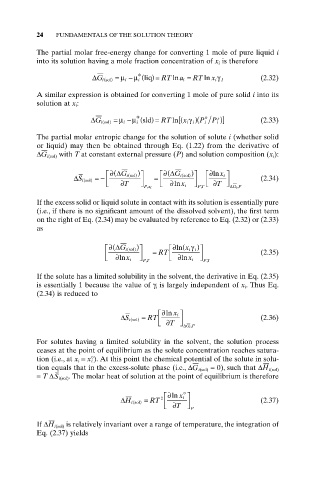
24 FUNDAMENTALS OF THE SOLUTION THEORY
The partial molar free-energy change for converting 1 mole of pure liquid i
into its solution having a mole fraction concentration of x i is therefore
*
( )
DG i sol) = m i - m i liq = RT ln a i = RT ln x i g I (2.32)
(
A similar expression is obtained for converting 1 mole of pure solid i into its
solution at x i :
* o s
)
(
ln
DG i sol) = m i - m i sld = RT [ x i ( g i )( P i P i )] (2.33)
(
The partial molar entropic change for the solution of solute i (whether solid
or liquid) may then be obtained through Eq. (1.22) from the derivative of
DG i(sol) with T at constant external pressure (P) and solution composition (x i ):
È ( ∂ DG i sol) ) ˘ È ( ∂ DG i sol) ) ˘ È ∂ln x i ˘
(
(
DS i sol) =- Í ˙ = Í ˙ (2.34)
(
Î ∂ T ˚ Px i , Î ∂ln x i ˚ PT , Í Î ∂ T ˚ ˙ DGP , i
If the excess solid or liquid solute in contact with its solution is essentially pure
(i.e., if there is no significant amount of the dissolved solvent), the first term
on the right of Eq. (2.34) may be evaluated by reference to Eq. (2.32) or (2.33)
as
∂ (
(
È ∂ DG i sol) ) ˘ È ln x i g i ) ˘
(
Í ˙ = RT Í ˙ (2.35)
ln
ln
Î ∂ x i ˚ PT , Î ∂ x i ˚ PT ,
If the solute has a limited solubility in the solvent, the derivative in Eq. (2.35)
is essentially 1 because the value of g i is largely independent of x i. Thus Eq.
(2.34) is reduced to
È ∂ln x i ˘
DS i sol) = RT Í Î ∂ T ˚ ˙ DGP , i (2.36)
(
For solutes having a limited solubility in the solvent, the solution process
ceases at the point of equilibrium as the solute concentration reaches satura-
tion (i.e., at x i = x° i ). At this point the chemical potential of the solute in solu-
tion equals that in the excess-solute phase (i.e., DG i(sol) = 0), such that DH i(sol)
= T DS i(sol). The molar heat of solution at the point of equilibrium is therefore
o
È∂ln x i ˘
DH i sol) = RT 2 Í Î ∂ T ˚ ˙ P (2.37)
(
If DH i(sol) is relatively invariant over a range of temperature, the integration of
Eq. (2.37) yields