Page 59 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 59
50 FUNDAMENTALS OF THE ADSORPTION THEORY
Brunauer (1945), when an adsorbate penetrates the interior of the solid,
it either dissolves in the solid to form a solution or reacts with the solid to
form a new compound. We shall see in Chapter 6 that the uptake of polar
vapors or liquids by soils and certain minerals is eminently consistent with this
expectation.
4.7 ISOSTERIC HEAT OF ADSORPTION
Because the adsorbent surface is commonly energetically heterogeneous, the
exothermic heat of adsorption of a vapor (or a solute) usually varies with the
amount adsorbed. To account for the variation in adsorption heat, the isos-
teric heats of adsorption at some fixed adsorbate loadings are determined from
the equilibrium vapor pressures (or solute concentrations) of the isotherms
at different temperatures with the aid of the Clausius–Clapeyron equation.
Although the concept of isosteric heat is originally intended for adsorption
systems, it has been extended to nonadsorption systems (e.g., partition) to elu-
cidate whether a concentration-dependent heat effect occurs with the system.
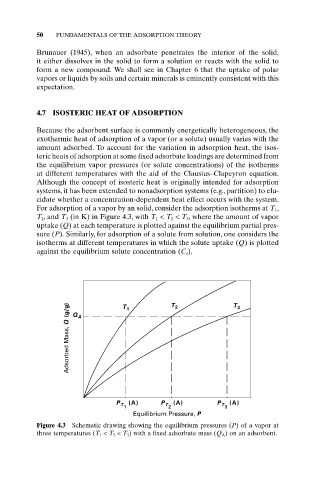
For adsorption of a vapor by an solid, consider the adsorption isotherms at T 1,
T 2, and T 3 (in K) in Figure 4.3, with T 1 < T 2 < T 3, where the amount of vapor
uptake (Q) at each temperature is plotted against the equilibrium partial pres-
sure (P). Similarly, for adsorption of a solute from solution, one considers the
isotherms at different temperatures in which the solute uptake (Q) is plotted
against the equilibrium solute concentration (C e).
Adsorbed Mass, Q (g/g) Q A T 1 T 2 T 3
P T (A) P T (A) P T (A)
1 2 3
Equilibrium Pressure, P
Figure 4.3 Schematic drawing showing the equilibrium pressures (P) of a vapor at
three temperatures (T 1 < T 2 < T 3 ) with a fixed adsorbate mass (Q A ) on an adsorbent.