Page 55 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 55
46 FUNDAMENTALS OF THE ADSORPTION THEORY
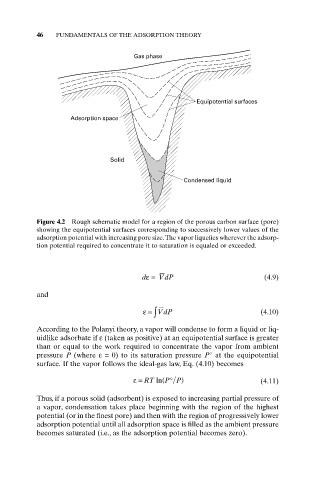
Gas phase
Equipotential surfaces
Adsorption space
Solid
Condensed liquid
Figure 4.2 Rough schematic model for a region of the porous carbon surface (pore)
showing the equipotential surfaces corresponding to successively lower values of the
adsorption potential with increasing pore size.The vapor liquefies wherever the adsorp-
tion potential required to concentrate it to saturation is equaled or exceeded.
de= V dP (4.9)
and
Ú
e= VdP (4.10)
According to the Polanyi theory, a vapor will condense to form a liquid or liq-
uidlike adsorbate if e (taken as positive) at an equipotential surface is greater
than or equal to the work required to concentrate the vapor from ambient
pressure P (where e= 0) to its saturation pressure P° at the equipotential
surface. If the vapor follows the ideal-gas law, Eq. (4.10) becomes
e= RT ln (P ∞ P ) (4.11)
Thus, if a porous solid (adsorbent) is exposed to increasing partial pressure of
a vapor, condensation takes place beginning with the region of the highest
potential (or in the finest pore) and then with the region of progressively lower
adsorption potential until all adsorption space is filled as the ambient pressure
becomes saturated (i.e., as the adsorption potential becomes zero).