Page 50 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 50
LANGMUIR ADSORPTION ISOTHERM 41
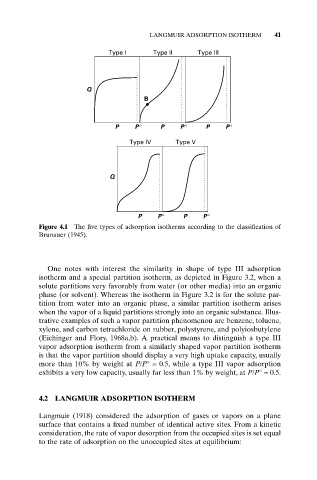
Type I Type II Type III
Q
B
P P° P P° P P°
Type IV Type V
Q
P P° P P°
Figure 4.1 The five types of adsorption isotherms according to the classification of
Brunauer (1945).
One notes with interest the similarity in shape of type III adsorption
isotherm and a special partition isotherm, as depicted in Figure 3.2, when a
solute partitions very favorably from water (or other media) into an organic
phase (or solvent). Whereas the isotherm in Figure 3.2 is for the solute par-
tition from water into an organic phase, a similar partition isotherm arises
when the vapor of a liquid partitions strongly into an organic substance. Illus-
trative examples of such a vapor partition phenomenon are benzene, toluene,
xylene, and carbon tetrachloride on rubber, polystyrene, and polyiosbutylene
(Eichinger and Flory, 1968a,b). A practical means to distinguish a type III
vapor adsorption isotherm from a similarly shaped vapor partition isotherm
is that the vapor partition should display a very high uptake capacity, usually
more than 10% by weight at P/P° = 0.5, while a type III vapor adsorption
exhibits a very low capacity, usually far less than 1% by weight, at P/P° = 0.5.
4.2 LANGMUIR ADSORPTION ISOTHERM
Langmuir (1918) considered the adsorption of gases or vapors on a plane
surface that contains a fixed number of identical active sites. From a kinetic
consideration, the rate of vapor desorption from the occupied sites is set equal
to the rate of adsorption on the unoccupied sites at equilibrium: