Page 53 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 53
44 FUNDAMENTALS OF THE ADSORPTION THEORY
The model sets a theoretical basis for calculating the surface area of the solid.
The theory was derived on the assumptions that (1) the Langmuir equation
applies to each adsorbed layer (i.e., the surface has uniform and localized sites
so that there is no interference in adsorption between neighboring sites); (2)
the adsorption and desorption occur only onto and from the exposed layer
surfaces; (3) at solid–vapor equilibrium, the rate of adsorption onto the ith
layer is balanced by the rate of desorption from the (i + 1)th layer; and (4) the
molar heat of adsorption for the first layer is considered to be higher than for
the succeeding layers, the latter assumed to be equal to the heat of liquefac-
tion of the vapor. These considerations lead to an isotherm of the form
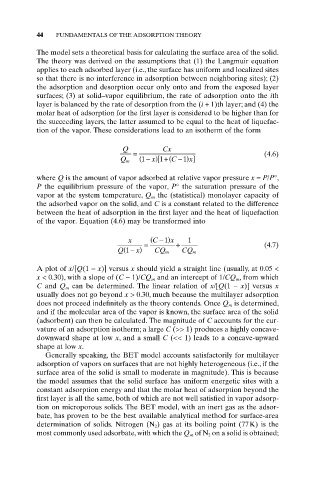
Q Cx
= (4.6)
x) +(
Q m (1 - [1 C - ) 1 x]
where Q is the amount of vapor adsorbed at relative vapor pressure x = P/P°,
P the equilibrium pressure of the vapor, P° the saturation pressure of the
vapor at the system temperature, Q m the (statistical) monolayer capacity of
the adsorbed vapor on the solid, and C is a constant related to the difference
between the heat of adsorption in the first layer and the heat of liquefaction
of the vapor. Equation (4.6) may be transformed into
-
x ( C 1) x 1
= + (4.7)
(
Q 1 - x) CQ m CQ m
A plot of x/[Q(1 - x)] versus x should yield a straight line (usually, at 0.05 <
x < 0.30), with a slope of (C - 1)/CQ m and an intercept of 1/CQ m, from which
C and Q m can be determined. The linear relation of x/[Q(1 - x)] versus x
usually does not go beyond x > 0.30, much because the multilayer adsorption
does not proceed indefinitely as the theory contends. Once Q m is determined,
and if the molecular area of the vapor is known, the surface area of the solid
(adsorbent) can then be calculated. The magnitude of C accounts for the cur-
vature of an adsorption isotherm; a large C (>> 1) produces a highly concave-
downward shape at low x, and a small C (<< 1) leads to a concave-upward
shape at low x.
Generally speaking, the BET model accounts satisfactorily for multilayer
adsorption of vapors on surfaces that are not highly heterogeneous (i.e., if the
surface area of the solid is small to moderate in magnitude). This is because
the model assumes that the solid surface has uniform energetic sites with a
constant adsorption energy and that the molar heat of adsorption beyond the
first layer is all the same, both of which are not well satisfied in vapor adsorp-
tion on microporous solids. The BET model, with an inert gas as the adsor-
bate, has proven to be the best available analytical method for surface-area
determination of solids. Nitrogen (N 2 ) gas at its boiling point (77K) is the
most commonly used adsorbate, with which the Q m of N 2 on a solid is obtained;