Page 194 - Petroleum and Gas Field Processing
P. 194
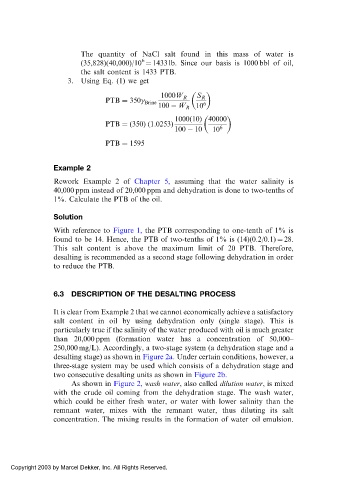
The quantity of NaCl salt found in this mass of water is
6
(35,828)(40,000)/10 ¼ 1433 lb. Since our basis is 1000 bbl of oil,
the salt content is 1433 PTB.
3. Using Eq. (1) we get
1000W R S R
PTB ¼ 350
Brine 6
100 W R 10
1000ð10Þ 40000
PTB ¼ð350Þð1:0253Þ 6
100 10 10
PTB ¼ 1595
Example 2
Rework Example 2 of Chapter 5, assuming that the water salinity is
40,000 ppm instead of 20,000 ppm and dehydration is done to two-tenths of
1%. Calculate the PTB of the oil.
Solution
With reference to Figure 1, the PTB corresponding to one-tenth of 1% is
found to be 14. Hence, the PTB of two-tenths of 1% is (14)(0.2/0.1) ¼ 28.
This salt content is above the maximum limit of 20 PTB. Therefore,
desalting is recommended as a second stage following dehydration in order
to reduce the PTB.
6.3 DESCRIPTION OF THE DESALTING PROCESS
It is clear from Example 2 that we cannot economically achieve a satisfactory
salt content in oil by using dehydration only (single stage). This is
particularly true if the salinity of the water produced with oil is much greater
than 20,000 ppm (formation water has a concentration of 50,000–
250,000 mg/L). Accordingly, a two-stage system (a dehydration stage and a
desalting stage) as shown in Figure 2a. Under certain conditions, however, a
three-stage system may be used which consists of a dehydration stage and
two consecutive desalting units as shown in Figure 2b.
As shown in Figure 2, wash water, also called dilution water, is mixed
with the crude oil coming from the dehydration stage. The wash water,
which could be either fresh water, or water with lower salinity than the
remnant water, mixes with the remnant water, thus diluting its salt
concentration. The mixing results in the formation of water–oil emulsion.
Copyright 2003 by Marcel Dekker, Inc. All Rights Reserved.