Page 235 - Petrophysics 2E
P. 235
208 PETROPHYSICS: RESERVOIR ROCK PROPERTIES
grams per liter of sodium chloride. One grain per gallon is approximately
equal to 17.2 ppm or 17.14 x g/L. Inasmuch as sodium chloride
is the most common salt present in the formation water, ionic
concentration of other dissolved salts is generally converted to the
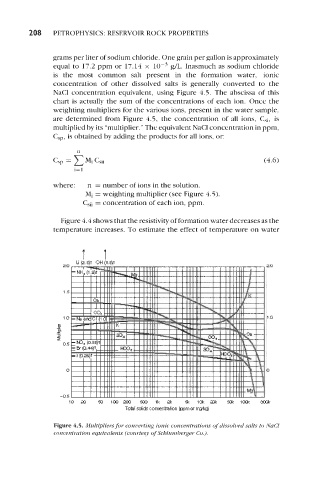
NaCl concentration equivalent, using Figure 4.5. The abscissa of this
chart is actually the sum of the concentrations of each ion. Once the
weighting multipliers for the various ions, present in the water sample,
are determined from Figure 4.5, the concentration of all ions, Csi, is
multiplied by its “multiplier.” The equivalent NaCl concentration in ppm,
C,,, is obtained by adding the products for all ions, or:
n
(4.6)
i= 1
where: n = number of ions in the solution.
Mi = weighting multiplier (see Figure 4.5).
Csii = concentration of each ion, ppm.
Figure 4.4 shows that the resistivity of formation water decreases as the
temperature increases. To estimate the effect of temperature on water
Figure 4.5. Multipliers for converting ionic concentrations of dissolved salts to NaCl
concentration equivalents (courtesy of Schlurnberger Co.).