Page 28 - Petrophysics 2E
P. 28
MINERAL CONSTITUENTS OF ROCKS-A REVIEW 3
preferential wetting of the surface by oil through Lewis acid-base
type reactions between the polar organic compounds in crude oils
and the transition metals exposed in the pores [3]. The high surface
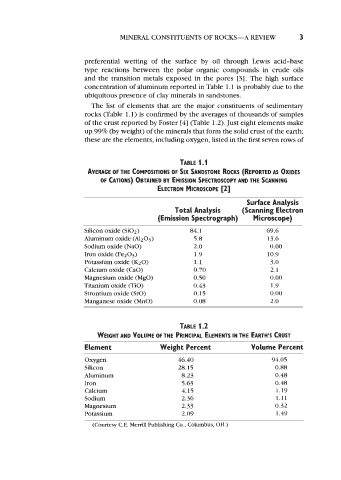
concentration of aluminum reported in Table 1.1 is probably due to the
ubiquitous presence of clay minerals in sandstones.
The list of elements that are the major constituents of sedimentary
rocks (Table 1.1) is confirmed by the averages of thousands of samples
of the crust reported by Foster [4] (Table 1.2). Just eight elements make
up 99% (by weight) of the minerals that form the solid crust of the earth;
these are the elements, including oxygen, listed in the first seven rows of
TABLE 1.1
AVERAGE OF THE COMPOSITIONS OF SIX SANDSTONE ROCKS (REPORTED AS OXIDES
OF CATIONS) OBTAINED BY EMISSION SPECTROSCOPY AND THE SCANNING
[2]
ELECTRON MICROSCOPE
Surface Analysis
Total Analysis (Scanning Electron
(Emission Spectrograph) Microscope)
Silicon oxide (Si02) 84.1 69.6
Aluminum oxide (Al2O3) 5.8 13.6
Sodium oxide (NaO) 2.0 0.00
Iron oxide (FezO3) 1.9 10.9
Potassium oxide (K20) 1.1 3.0
Calcium oxide (CaO) 0.70 2.1
Magnesium oxide (MgO) 0.50 0.00
Titanium oxide (TiO) 0.43 1.9
Strontium oxide (SrO) 0.15 0.00
Manganese oxide (MnO) 0.08 2.0
TABLE 1.2
WEIGHT AND VOLUME OF THE PRINCIPAL ELEMENTS THE EARTH’S CRUST
IN
Element Weight Percent Volume Percent
Oxygen 46.40 94.05
Silicon 28.15 0.88
Aluminum 8.23 0.48
Iron 5.63 0.48
Calcium 4.15 1.19
Sodium 2.36 1.11
Magnesium 2.33 0.32
Potassium 2.09 1.49
(Courtesy C.E. Merrill Publishing Co., Columbus, OH.)