Page 237 - Petrophysics
P. 237
2 10 PETROPHYSICS: RESERVOIR ROCK PROPERTIES
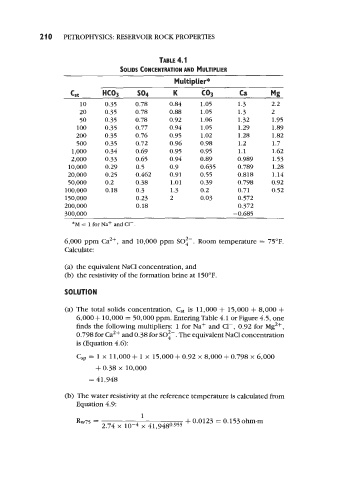
TABLE 4.1
SOLIDS CONCENTRATION AND MULTIPLIER
Multiplier*
cst HCO3 so4 K co3 Ca Mg
10 0.35 0.78 0.84 1.05 1.3 2.2
20 0.35 0.78 0.88 1.05 1.3 2
50 0.35 0.78 0.92 1.06 1.32 1.95
100 0.35 0.77 0.94 1.05 1.29 1.89
200 0.35 0.76 0.95 1.02 1.28 1 .82
500 0.35 0.72 0.96 0.98 1.2 1.7
1,000 0.34 0.69 0.95 0.95 1.1 1.62
2,000 0.33 0.65 0.94 0.89 0.989 1.53
10,000 0.29 0.5 0.9 0.635 0.789 1.28
20,000 0.25 0.462 0.91 0.55 0.818 1.14
50,000 0.2 0.38 1.01 0.39 0.798 0.92
100,000 0.18 0.3 1.3 0.2 0.71 0.52
150,000 0.23 2 0.03 0.572
200,000 0.18 0.372
300,000 -0.685
*M = 1 for Na+ and C1-.
6,000 ppm Ca2+, and 10,000 ppm SO:-. Room temperature = 75°F.
Calculate :
(a) the equivalent NaCl concentration, and
(b) the resistivity of the formation brine at 150°F.
SOLUTION
(a) The total solids concentration, CSt is 11,000 + 15,000 + 8,000 +
6,000 + 10,000 = 50,000 pprn. Entering Table 4.1 or Figure 4.5, one
finds the following multipliers: 1 for Na+ and C1-, 0.92 for Mg2+,
0.798 for Ca2+ and 0.38 for SO:-. The equivalent NaCl concentration
is (Equation 4.6):
C, = 1 x 11,000 + 1 x 15,000 + 0.92 x 8,000 + 0.798 x 6,000
+ 0.38 x 10,000
= 41,948
(b) The water resistivity at the reference temperature is calculated from
Equation 4.9:
1
Rw75 = + 0.0123 = 0.153 ohm-m
2.74 x lo-* x 41,948O.955