Page 86 - Petrophysics
P. 86
60 PETROPHYSICS: RESERVOIR ROCK PROPERTIES
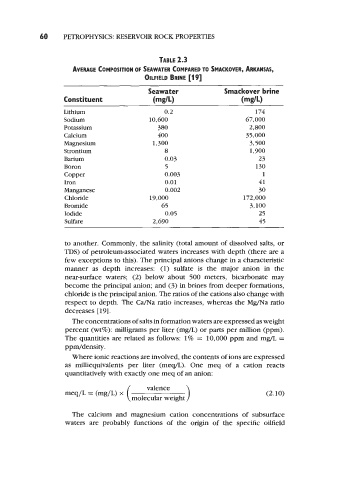
TABLE 2.3
AVERAGE COMPOSITION OF SEAWATER COMPARED TO SMACKOVER, ARKANSAS,
OILFIELD BRINE [19]
Seawater Smackover brine
Constituent (mg/L) (mg/L)
Lithium 0.2 174
Sodium 10,600 67,000
Potassium 380 2,800
Calcium 400 35,000
Magnesium 1,300 3,500
Strontium 8 1,900
Barium 0.03 23
Boron 5 130
Copper 0.003 1
Iron 0.01 41
Manganese 0.002 30
Chloride 19,000 172,000
Bromide 65 3,100
Iodide 0.05 25
Sulfate 2,690 45
to another. Commonly, the salinity (total amount of dissolved salts, or
TDS) of petroleum-associated waters increases with depth (there are a
few exceptions to this). The principal anions change in a characteristic
manner as depth increases: (1) sulfate is the major anion in the
near-surface waters; (2) below about 500 meters, bicarbonate may
become the principal anion; and (3) in brines from deeper formations,
chloride is the principal anion. The ratios of the cations also change with
respect to depth. The Ca/Na ratio increases, whereas the Mg/Na ratio
decreases [ 191.
The concentrations of salts in formation waters are expressed as weight
percent (wt%): milligrams per liter (mg/L) or parts per million (ppm).
The quantities are related as follows: 1% = 10,000 ppm and mg/L =
ppm/density .
Where ionic reactions are involved, the contents of ions are expressed
as milliequivalents per liter (meq/L). One meq of a cation reacts
quantitatively with exactly one meq of an anion:
valence
meq/L = (mg/L) x (2. 10)
molecular weight
The calcium and magnesium cation concentrations of subsurface
waters are probably functions of the origin of the specific oilfield