Page 91 - Petrophysics
P. 91
PROPERTIES OF SUBSURFACE FLUIDS
40.-1W 1%
l20*-212’ 6%
212‘400’ 10%
o 100 2o~aoo4oo
T.
PRESSWE CORRECTON FACTOR (1) MR
WATER VS T, T PREWYED A-BLE
To BRmEs BUT m)T COMRYD
EXPERlYWTAUY
VISCOSITY AT ELEVATED PRESSURE
Cp,T+C.T. fp, T
VISCOSITY (Po) AT 1 ATY
PRESSURE BELOW 212. AT
0.4
0.3
0.2
0.1
n
40 60 80 100 120 140 160 80 200 220 240 260 280 300 320 340 360 380 400
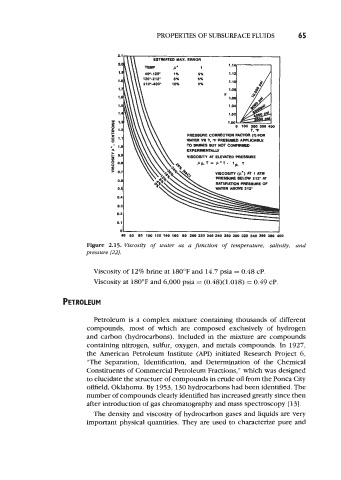
Figure 2.15. Viscosity of water as a function of temperature, salinity, and
pressure [22J
Viscosity of 12% brine at 180°F and 14.7 psia = 0.48 cP.
Viscosity at 180°F and 6,000 psia = (0.48)(1.018) = 0.49 cP.
PETROLEUM
Petroleum is a complex mixture containing thousands of different
compounds, most of which are composed exclusively of hydrogen
and carbon (hydrocarbons). Included in the mixture are compounds
containing nitrogen, sulfur, oxygen, and metals compounds. In 1927,
the American Petroleum Institute (MI) initiated Research Project 6,
“The Separation, Identification, and Determination of the Chemical
Constituents of Commercial Petroleum Fractions, ” which was designed
to elucidate the structure of compounds in crude oil from the Ponca City
oilfield, Oklahoma. By 1953, 130 hydrocarbons had been identified. The
number of compounds clearly identified has increased greatly since then
after introduction of gas chromatography and mass spectroscopy [ 131.
The density and viscosity of hydrocarbon gases and liquids are very
important physical quantities. They are used to characterize pure and