Page 23 - Photonics Essentials an introduction with experiments
P. 23
Electrons and Photons
Electrons and Photons 17
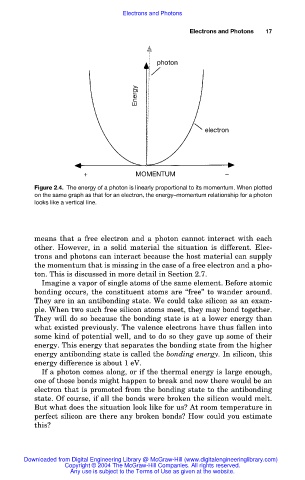
photon
Energy
electron
+ MOMENTUM –
Figure 2.4. The energy of a photon is linearly proportional to its momentum. When plotted
on the same graph as that for an electron, the energy–momentum relationship for a photon
looks like a vertical line.
means that a free electron and a photon cannot interact with each
other. However, in a solid material the situation is different. Elec-
trons and photons can interact because the host material can supply
the momentum that is missing in the case of a free electron and a pho-
ton. This is discussed in more detail in Section 2.7.
Imagine a vapor of single atoms of the same element. Before atomic
bonding occurs, the constituent atoms are “free” to wander around.
They are in an antibonding state. We could take silicon as an exam-
ple. When two such free silicon atoms meet, they may bond together.
They will do so because the bonding state is at a lower energy than
what existed previously. The valence electrons have thus fallen into
some kind of potential well, and to do so they gave up some of their
energy. This energy that separates the bonding state from the higher
energy antibonding state is called the bonding energy. In silicon, this
energy difference is about 1 eV.
If a photon comes along, or if the thermal energy is large enough,
one of those bonds might happen to break and now there would be an
electron that is promoted from the bonding state to the antibonding
state. Of course, if all the bonds were broken the silicon would melt.
But what does the situation look like for us? At room temperature in
perfect silicon are there any broken bonds? How could you estimate
this?
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2004 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.