Page 149 - Physical chemistry understanding our chemical world
P. 149
116 ENERGY AND THE FIRST LAW OF THERMODYNAMICS
O
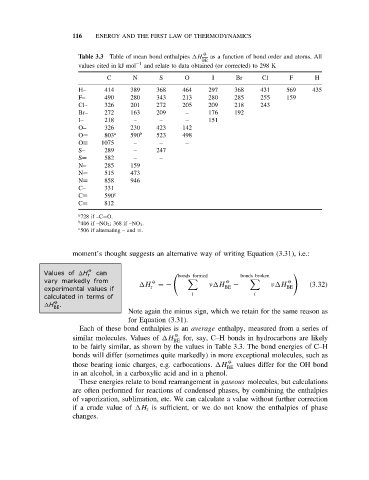
Table 3.3 Table of mean bond enthalpies H as a function of bond order and atoms. All
BE
values cited in kJ mol −1 and relate to data obtained (or corrected) to 298 K
C N S O I Br Cl F H
H– 414 389 368 464 297 368 431 569 435
F– 490 280 343 213 280 285 255 159
Cl– 326 201 272 205 209 218 243
Br– 272 163 209 – 176 192
I– 218 – – – 151
O– 326 230 423 142
O= 803 a 590 b 523 498
O≡ 1075 – – –
S– 289 – 247
S= 582 – –
N– 285 159
N= 515 473
N= 858 946
C– 331
C= 590 c
C≡ 812
a 728 if –C=O.
b 406 if –NO 2 ; 368 if –NO 3 .
c 506 if alternating – and =.
moment’s thought suggests an alternative way of writing Equation (3.31), i.e.:
O
Values of H r can
bonds formed bonds broken
vary markedly from H O =− ν H O − ν H O (3.32)
experimental values if r BE BE
i i
calculated in terms of
O
H .
BE
Note again the minus sign, which we retain for the same reason as
for Equation (3.31).
Each of these bond enthalpies is an average enthalpy, measured from a series of
O
similar molecules. Values of H BE for, say, C–H bonds in hydrocarbons are likely
to be fairly similar, as shown by the values in Table 3.3. The bond energies of C–H
bonds will differ (sometimes quite markedly) in more exceptional molecules, such as
those bearing ionic charges, e.g. carbocations. H O values differ for the OH bond
BE
in an alcohol, in a carboxylic acid and in a phenol.
These energies relate to bond rearrangement in gaseous molecules, but calculations
are often performed for reactions of condensed phases, by combining the enthalpies
of vaporization, sublimation, etc. We can calculate a value without further correction
if a crude value of H r is sufficient, or we do not know the enthalpies of phase
changes.