Page 146 - Physical chemistry understanding our chemical world
P. 146
ENTHALPY 113
O
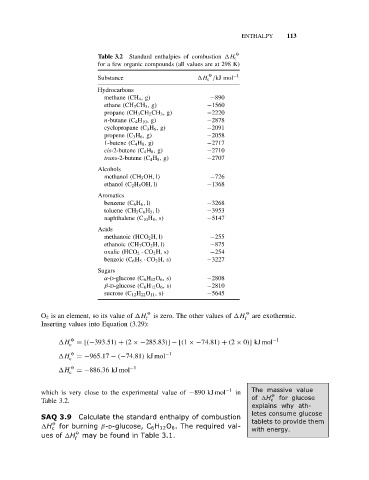
Table 3.2 Standard enthalpies of combustion H c
for a few organic compounds (all values are at 298 K)
O
Substance H c /kJ mol −1
Hydrocarbons
methane (CH 4 ,g) −890
ethane (CH 3 CH 3 ,g) −1560
propane (CH 3 CH 2 CH 3 ,g) −2220
n-butane (C 4 H 10 ,g) −2878
cyclopropane (C 3 H 6 ,g) −2091
propene (C 3 H 6 ,g) −2058
1-butene (C 4 H 8 ,g) −2717
cis-2-butene (C 4 H 8 ,g) −2710
trans-2-butene (C 4 H 8 ,g) −2707
Alcohols
methanol (CH 3 OH, l) −726
ethanol (C 2 H 5 OH, l) −1368
Aromatics
benzene (C 6 H 6 , l) −3268
toluene (CH 3 C 6 H 5 , l) −3953
naphthalene (C 10 H 8 ,s) −5147
Acids
methanoic (HCO 2 H, l) −255
ethanoic (CH 3 CO 2 H, l) −875
oxalic (HCO 2 · CO 2 H, s) −254
benzoic (C 6 H 5 · CO 2 H, s) −3227
Sugars
α-D-glucose (C 6 H 12 O 6 ,s) −2808
β-D-glucose (C 6 H 12 O 6 ,s) −2810
sucrose (C 12 H 22 O 11 ,s) −5645
O 2 is an element, so its value of H O is zero. The other values of H O are exothermic.
f f
Inserting values into Equation (3.29):
H c O = [(−393.51) + (2 ×−285.83)] − [(1 ×−74.81) + (2 × 0)]kJ mol −1
H O =−965.17 − (−74.81) kJ mol −1
c
H O =−886.36 kJ mol −1
c
which is very close to the experimental value of −890 kJ mol −1 in The massive value
O for glucose
Table 3.2. of H c
explains why ath-
letes consume glucose
SAQ 3.9 Calculate the standard enthalpy of combustion
tablets to provide them
O
H c for burning β-D-glucose, C 6 H 12 O 6 . The required val- with energy.
ues of H O may befound in Table3.1.
f