Page 266 - Physical chemistry understanding our chemical world
P. 266
6
Acids and Bases
Introduction
Equilibria involving acids and bases are discussed from within the Lowry–Brønsted
theory, which defines an acid as a proton donor and a base as a proton acceptor (or
‘abstracter’). The additional concept of pH is then introduced. ‘Strong’ and ‘weak’
acids are discussed in terms of the acidity constant K a , and then conjugate acids and
bases are identified.
Acid–base buffers comprise both a weak acid or base and its respective salt. Cal-
culations with buffers employing the Henderson–Hasselbach equation are introduced
and evaluated, thereby allowing the calculation of the pH of a buffer. Next, titrations
and pH indicators are discussed, and their modes of action placed into context.
6.1 Properties of Lowry–Brønsted acids
and bases
Why does vinegar taste sour?
The Lowry–Brønsted theory of acids
We instantly experience a sour, bitter taste when consuming anything containing
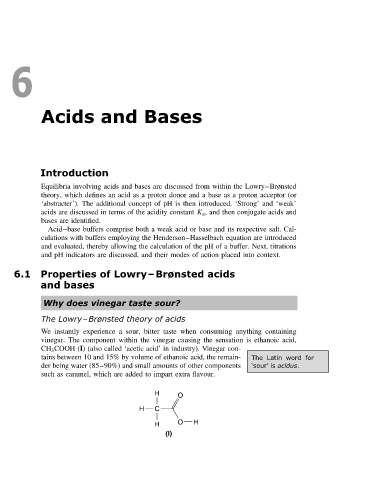
vinegar. The component within the vinegar causing the sensation is ethanoic acid,
CH 3 COOH (I) (also called ‘acetic acid’ in industry). Vinegar con-
tains between 10 and 15% by volume of ethanoic acid, the remain- The Latin word for
der being water (85–90%) and small amounts of other components ‘sour’ is acidus.
such as caramel, which are added to impart extra flavour.
H O
H C
H O H
(I)