Page 69 - Physical chemistry understanding our chemical world
P. 69
36 INTRODUCTION TO PHYSICAL CHEMISTRY
100 K
Energy distribution 200 K 300 K 500 K
700 K
1000 K
500 1000 1500
Velocity v /m s −1
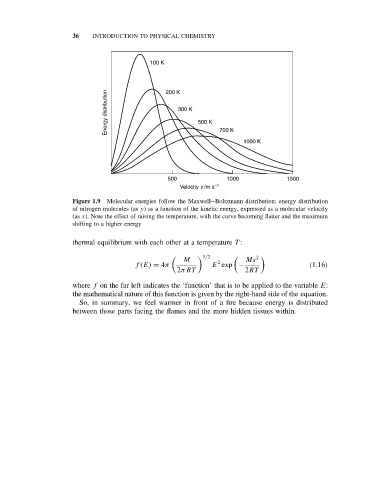
Figure 1.9 Molecular energies follow the Maxwell–Boltzmann distribution: energy distribution
of nitrogen molecules (as y) as a function of the kinetic energy, expressed as a molecular velocity
(as x). Note the effect of raising the temperature, with the curve becoming flatter and the maximum
shifting to a higher energy
thermal equilibrium with each other at a temperature T :
3/2 2
M 2 Ms
f(E) = 4π E exp − (1.16)
2πRT 2RT
where f on the far left indicates the ‘function’ that is to be applied to the variable E:
the mathematical nature of this function is given by the right-hand side of the equation.
So, in summary, we feel warmer in front of a fire because energy is distributed
between those parts facing the flames and the more hidden tissues within.