Page 73 - Physical chemistry understanding our chemical world
P. 73
40 INTRODUCING INTERACTIONS AND BONDS
We perceive this lower energy as a cooler temperature, meaning
Energy is never lost
that the water vapour in a steam-filled bathroom will cool down;
or gained, only trans- conversely, the mirror (and walls) become warmer as they receive
ferred or converted;
the energy that was previously possessed by the steam. These
see Chapter 3.
changes in the temperatures of gas and mirror occur in a com-
plementary sense, so no energy is gained or lost.
These changes in temperature represent a macroscopic proof that
We generally assume microscopic processes do occur. Indeed, it is difficult to envisage
that all particles in an a transfer of energy between the gas particles with the cold mirror
ideal gas do not inter- without these microscopic interactions.
act, meaning that the We spent quite a lot of time looking at the concept of an ideal gas
gas obeys the ideal-gas in Chapter 1. The simplest definition of an ideal gas is that it obeys
equation. This assump- the ideal-gas equation (Equation (1.13)). Most gases can be con-
tion is sometimes poor.
sidered as ideal most of the time. The most common cause of a gas
disobeying the ideal-gas equation is the formation of interactions,
and the results of intermolecular collisions.
How does a liquid-crystal display work?
Electronegativity and electropositivity
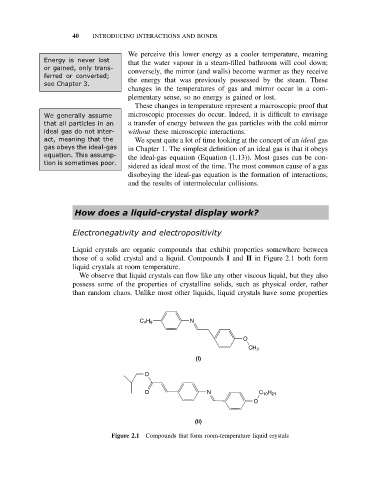
Liquid crystals are organic compounds that exhibit properties somewhere between
those of a solid crystal and a liquid. Compounds I and II in Figure 2.1 both form
liquid crystals at room temperature.
We observe that liquid crystals can flow like any other viscous liquid, but they also
possess some of the properties of crystalline solids, such as physical order, rather
than random chaos. Unlike most other liquids, liquid crystals have some properties
C 4 H 9 N
O
CH 3
(I)
O
O N C 10 H 21
O
(II)
Figure 2.1 Compounds that form room-temperature liquid crystals