Page 98 - Physical chemistry understanding our chemical world
P. 98
CREATING FORMAL CHEMICAL BONDS 65
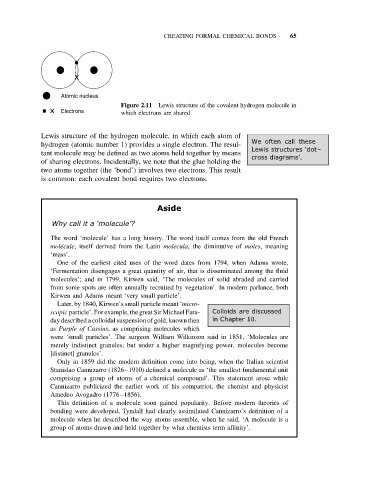
Atomic nucleus
Figure 2.11 Lewis structure of the covalent hydrogen molecule in
Electrons which electrons are shared
Lewis structure of the hydrogen molecule, in which each atom of
We often call these
hydrogen (atomic number 1) provides a single electron. The resul-
Lewis structures ‘dot–
tant molecule may be defined as two atoms held together by means
cross diagrams’.
of sharing electrons. Incidentally, we note that the glue holding the
two atoms together (the ‘bond’) involves two electrons. This result
is common: each covalent bond requires two electrons.
Aside
Why call it a ‘molecule’?
The word ‘molecule’ has a long history. The word itself comes from the old French
mol´ecule, itself derived from the Latin molecula, the diminutive of moles, meaning
‘mass’.
One of the earliest cited uses of the word dates from 1794, when Adams wrote,
‘Fermentation disengages a great quantity of air, that is disseminated among the fluid
molecules’; and in 1799, Kirwen said, ‘The molecules of solid abraded and carried
from some spots are often annually recruited by vegetation’. In modern parlance, both
Kirwen and Adams meant ‘very small particle’.
Later, by 1840, Kirwen’s small particle meant ‘micro-
scopic particle’. For example, the great Sir Michael Fara- Colloids are discussed
day described a colloidal suspension of gold, known then in Chapter 10.
as Purple of Cassius, as comprising molecules which
were ‘small particles’. The surgeon William Wilkinson said in 1851, ‘Molecules are
merely indistinct granules; but under a higher magnifying power, molecules become
[distinct] granules’.
Only in 1859 did the modern definition come into being, when the Italian scientist
Stanislao Cannizarro (1826–1910) defined a molecule as ‘the smallest fundamental unit
comprising a group of atoms of a chemical compound’. This statement arose while
Cannizarro publicized the earlier work of his compatriot, the chemist and physicist
Amedeo Avogadro (1776–1856).
This definition of a molecule soon gained popularity. Before modern theories of
bonding were developed, Tyndall had clearly assimilated Cannizarro’s definition of a
molecule when he described the way atoms assemble, when he said, ‘A molecule is a
group of atoms drawn and held together by what chemists term affinity’.