Page 103 - Physical chemistry understanding our chemical world
P. 103
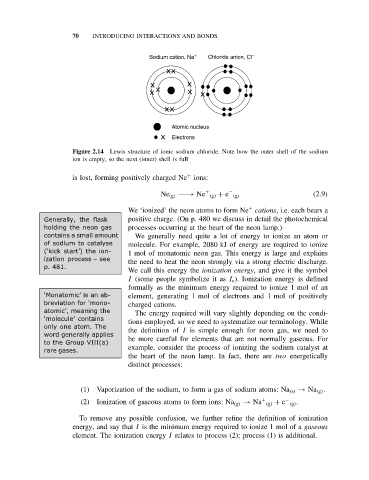
70 INTRODUCING INTERACTIONS AND BONDS
+ −
Sodium cation, Na Chloride anion, Cl
Atomic nucleus
Electrons
Figure 2.14 Lewis structure of ionic sodium chloride. Note how the outer shell of the sodium
ion is empty, so the next (inner) shell is full
+
is lost, forming positively charged Ne ions:
+ −
Ne (g) −−→ Ne (g) + e (g) (2.9)
+
We ‘ionized’ the neon atoms to form Ne cations, i.e. each bears a
Generally, the flask positive charge. (On p. 480 we discuss in detail the photochemical
holding the neon gas processes occurring at the heart of the neon lamp.)
contains a small amount We generally need quite a lot of energy to ionize an atom or
of sodium to catalyse molecule. For example, 2080 kJ of energy are required to ionize
(‘kick start’) the ion- 1 mol of monatomic neon gas. This energy is large and explains
ization process – see the need to heat the neon strongly via a strong electric discharge.
p. 481.
We call this energy the ionization energy, and give it the symbol
I (some people symbolize it as I e ). Ionization energy is defined
formally as the minimum energy required to ionize 1 mol of an
‘Monatomic’ is an ab- element, generating 1 mol of electrons and 1 mol of positively
breviation for ‘mono- charged cations.
atomic’, meaning the The energy required will vary slightly depending on the condi-
‘molecule’ contains tions employed, so we need to systematize our terminology. While
only one atom. The the definition of I is simple enough for neon gas, we need to
word generally applies be more careful for elements that are not normally gaseous. For
to the Group VIII(a)
rare gases. example, consider the process of ionizing the sodium catalyst at
the heart of the neon lamp. In fact, there are two energetically
distinct processes:
(1) Vaporization of the sodium, to form a gas of sodium atoms: Na (s) → Na (g) .
+
−
(2) Ionization of gaseous atoms to form ions: Na (g) → Na (g) + e (g) .
To remove any possible confusion, we further refine the definition of ionization
energy, and say that I is the minimum energy required to ionize 1 mol of a gaseous
element. The ionization energy I relates to process (2); process (1) is additional.