Page 104 - Physical chemistry understanding our chemical world
P. 104
CREATING FORMAL CHEMICAL BONDS 71
Table 2.8 Ionization energies I (n) . For convenience, the figures in the table are given in MJ mol −1
rather than the more usual kJ mol −1 to emphasize their magnitudes
Element I (1) I (2) I (3) I (4) I (5) I (6) I (7) I (8) I (9) I (10)
Hydrogen 1.318
Helium 2.379 5.257
Lithium 0.526 7.305 11.822
Beryllium 0.906 1.763 14.855 21.013
Boron 0.807 2.433 3.666 25.033 32.834
Carbon 1.093 2.359 4.627 6.229 37.838 47.285
Nitrogen 1.407 2.862 4.585 7.482 9.452 53.274 64.368
Oxygen 1.320 3.395 5.307 7.476 10.996 13.333 71.343 84.086
Fluorine 1.687 3.381 6.057 8.414 11.029 15.171 17.874 92.047 106.443
Neon 2.097 3.959 6.128 9.376 12.184 15.245 20.006 23.076 115.389 131.442
2500
−1 2000
First ionization energy I/kJ mol 1500
1000
500
0
0 20 40 60 80 100
Atomic number
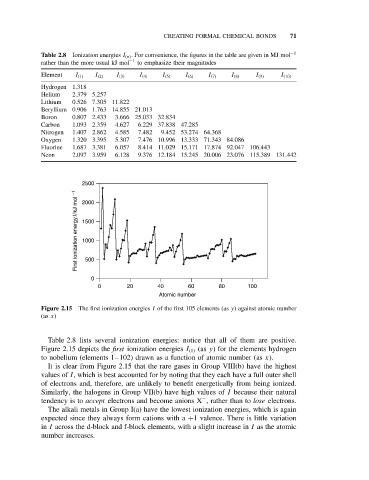
Figure 2.15 The first ionization energies I of the first 105 elements (as y) against atomic number
(as x)
Table 2.8 lists several ionization energies: notice that all of them are positive.
Figure 2.15 depicts the first ionization energies I (1) (as y) for the elements hydrogen
to nobelium (elements 1–102) drawn as a function of atomic number (as x).
It is clear from Figure 2.15 that the rare gases in Group VIII(b) have the highest
values of I, which is best accounted for by noting that they each have a full outer shell
of electrons and, therefore, are unlikely to benefit energetically from being ionized.
Similarly, the halogens in Group VII(b) have high values of I because their natural
tendency is to accept electrons and become anions X , rather than to lose electrons.
−
The alkali metals in Group I(a) have the lowest ionization energies, which is again
expected since they always form cations with a +1 valence. There is little variation
in I across the d-block and f-block elements, with a slight increase in I as the atomic
number increases.