Page 211 - Physical Chemistry
P. 211
lev38627_ch06.qxd 3/3/08 10:07 AM Page 192
192
Chapter 6 With simultaneous equilibria, it is often simpler to use conservation-of-matter
Reaction Equilibrium in Ideal Gas conditions for each element instead of using the extents of reaction. The number of
Mixtures
moles of each element are:
n n CH 4 n CO 2 n , n 4n CH 4 2n H 2 O 2n , n n H 2 O 2n CO 2 n CO
CO
H
C
O
H 2
Using the initial composition, we have n 1 1 2 4, n 8, and n 5
O
C
H
throughout the reaction. At 900 K, the NIST-JANAF tables (Sec. 5.9) G° data give
f
the equilibrium constants for the reactions as K° 1.30 and K° 2.99. We shall
P,1
P,2
find the equilibrium composition at a fixed pressure of 0.01 bar. We have five unknowns,
the numbers of moles of each of the five species in the reactions. The five equations
to be satisfied are the two equilibrium conditions and the three conservation-of-moles
conditions for each element.
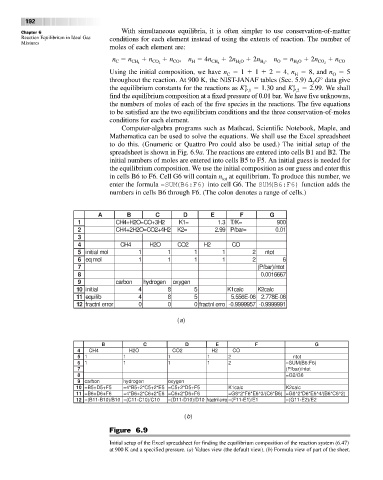
Computer-algebra programs such as Mathcad, Scientific Notebook, Maple, and
Mathematica can be used to solve the equations. We shall use the Excel spreadsheet
to do this. (Gnumeric or Quattro Pro could also be used.) The initial setup of the
spreadsheet is shown in Fig. 6.9a. The reactions are entered into cells B1 and B2. The
initial numbers of moles are entered into cells B5 to F5. An initial guess is needed for
the equilibrium composition. We use the initial composition as our guess and enter this
in cells B6 to F6. Cell G6 will contain n at equilibrium. To produce this number, we
tot
enter the formula =SUM(B6:F6) into cell G6. The SUM(B6:F6) function adds the
numbers in cells B6 through F6. (The colon denotes a range of cells.)
A B C D E F G
1 CH4+H2O=CO+3H2 K1= 1.3 T/K= 900
2 CH4+2H2O=CO2+4H2 K2= 2.99 P/bar= 0.01
3
4 CH4 H2O CO2 H2 CO
5 initial mol 1 1 1 1 2 ntot
6 eq mol 1 1 1 1 2 6
7 (P/bar)/ntot
8 0.0016667
9 carbon hydrogen oxygen
10 initial 4 8 5 K1calc K2calc
11 equilib 4 8 5 5.556E-06 2.778E-06
12 fractnl error 0 0 0 fractnl erro -0.9999957 -0.9999991
(a)
B C D E F G
4 CH4 H2O CO2 H2 CO
5 1 1 1 1 2 ntot
6 1 1 1 1 2 =SUM(B6:F6)
7 (P/bar)/ntot
8 =G2/G6
9 carbon hydrogen oxygen
10 =B5+D5+F5 =4*B5+2*C5+2*E5 =C5+2*D5+F5 K1calc K2calc
11 =B6+D6+F6 =4*B6+2*C6+2*E6 =C6+2*D6+F6 =G8^2*F6*E6^3/(C6*B6) =G8^2*D6*E6^4/(B6*C6^2)
12 =(B11-B10)/B10 =(C11-C10)/C10 =(D11-D10)/D10 fractnl erro =(F11-E1)/E1 =(G11-E2)/E2
(b)
Figure 6.9
Initial setup of the Excel spreadsheet for finding the equilibrium composition of the reaction system (6.47)
at 900 K and a specified pressure. (a)Values view (the default view). (b)Formula view of part of the sheet.