Page 30 - Physical Chemistry
P. 30
lev38627_ch01.qxd 2/20/08 11:38 AM Page 11
11
Section 1.5
Ideal Gases
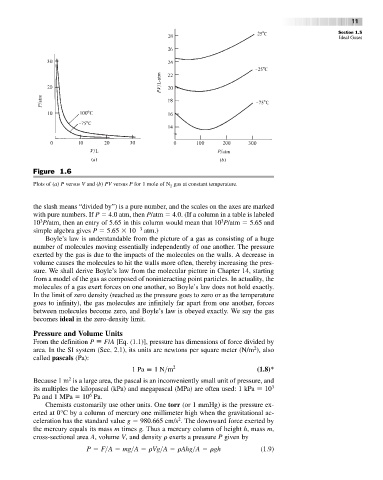
Figure 1.6
Plots of (a) P versus V and (b) PV versus P for 1 mole of N gas at constant temperature.
2
the slash means “divided by”) is a pure number, and the scales on the axes are marked
with pure numbers. If P 4.0 atm, then P/atm 4.0. (If a column in a table is labeled
3
3
10 P/atm, then an entry of 5.65 in this column would mean that 10 P/atm 5.65 and
simple algebra gives P 5.65 10 3 atm.)
Boyle’s law is understandable from the picture of a gas as consisting of a huge
number of molecules moving essentially independently of one another. The pressure
exerted by the gas is due to the impacts of the molecules on the walls. A decrease in
volume causes the molecules to hit the walls more often, thereby increasing the pres-
sure. We shall derive Boyle’s law from the molecular picture in Chapter 14, starting
from a model of the gas as composed of noninteracting point particles. In actuality, the
molecules of a gas exert forces on one another, so Boyle’s law does not hold exactly.
In the limit of zero density (reached as the pressure goes to zero or as the temperature
goes to infinity), the gas molecules are infinitely far apart from one another, forces
between molecules become zero, and Boyle’s law is obeyed exactly. We say the gas
becomes ideal in the zero-density limit.
Pressure and Volume Units
From the definition P F/A [Eq. (1.1)], pressure has dimensions of force divided by
2
area. In the SI system (Sec. 2.1), its units are newtons per square meter (N/m ), also
called pascals (Pa):
1 Pa 1 N>m 2 (1.8)*
2
Because 1 m is a large area, the pascal is an inconveniently small unit of pressure, and
its multiples the kilopascal (kPa) and megapascal (MPa) are often used: 1 kPa 10 3
6
Pa and 1 MPa 10 Pa.
Chemists customarily use other units. One torr (or 1 mmHg) is the pressure ex-
erted at 0°C by a column of mercury one millimeter high when the gravitational ac-
2
celeration has the standard value g 980.665 cm/s . The downward force exerted by
the mercury equals its mass m times g. Thus a mercury column of height h, mass m,
cross-sectional area A, volume V, and density r exerts a pressure P given by
P F>A mg>A rVg>A rAhg>A rgh (1.9)